Researchers Identify Key Players in Cell Division
New findings help answer foundational questions about cell genesis and survival
November 20, 2019
By Mario Aguilera
One of the most fundamental questions in biology lies at the heart of cell division. All living cells have the ability to regenerate through cell division, when a mother cell gives rise to two daughter cells. Although cell division has been studied extensively and is considered an essential process for the continuation and evolution of all living organisms, a central question remains: How are the mother and daughter cell components produced and fully maintained?
To help answer this question, scientists in Maho Niwa’s lab at the University of California San Diego have provided new insights into a key pathway that controls critical cell division mechanisms. The findings, published recently in Developmental Cell, also help illuminate the processes involved in failed cell division in human diseases, including cases ranging from viral infection to cancer.
Scientists have unraveled many important details of how cells ensure that all of their genomic information is accurately copied and divided into two dividing cells. Niwa and her colleagues study important cell constituents such as proteins, lipids and many types of organelles, which are specific compartments that feature key functions for cell survival. One such compartment, the endoplasmic reticulum, or ER, can produce a special group of important proteins that are sent outside of the cell. These include some of the most vital proteins to human health, including insulin, hormones and growth factors.
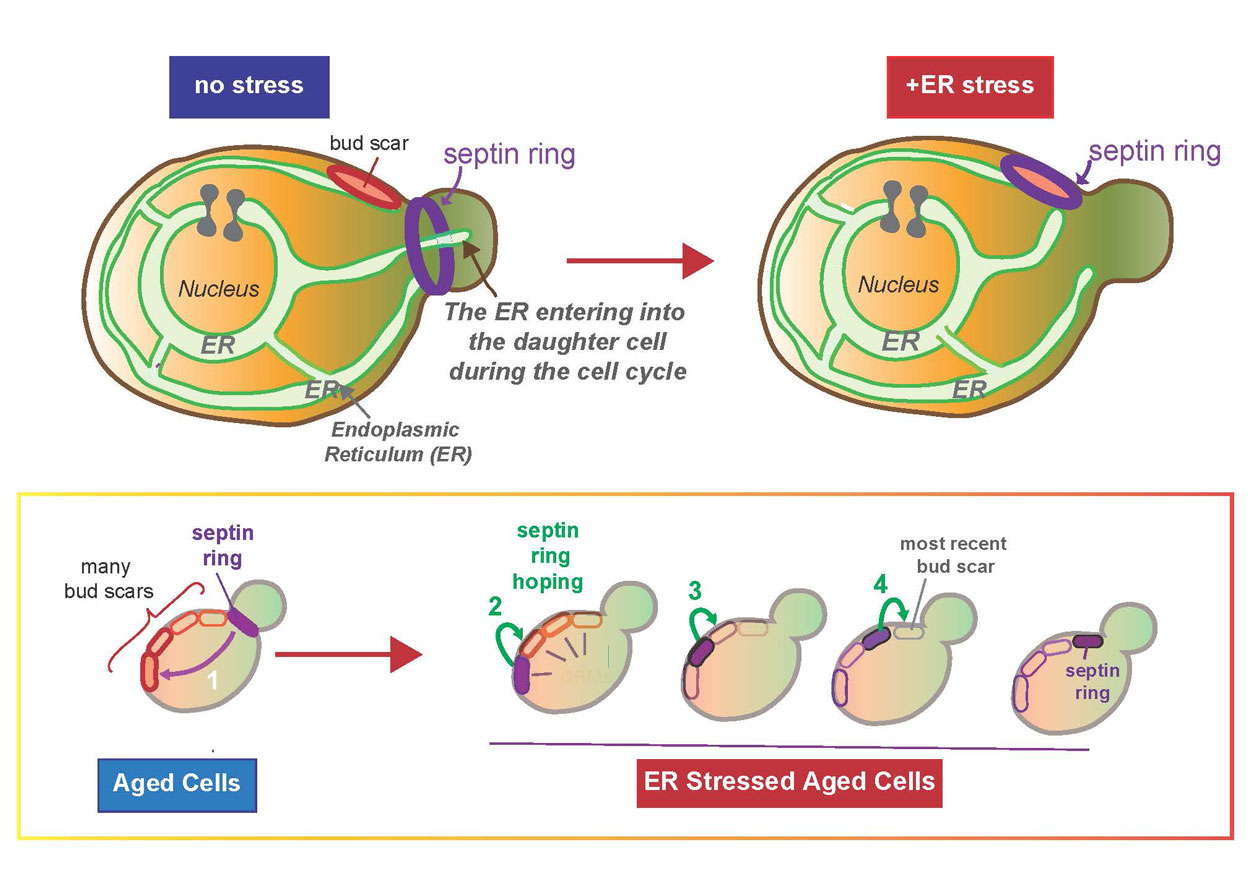
A key piece of this process, according to Niwa, is that the ER cannot be generated from scratch—it can only be inherited from the previous cell. In order to study such key inheritance mechanisms, Niwa and her colleagues use a simple model, the yeast Saccharomyces cerevisiae. Yeast’s simplicity can help answer specific questions, including how the mother cell makes sure that the newly born daughter cell receives sufficient ER to become a functioning cell that can ultimately give rise to her own progeny.
“How the ER is correctly inherited and what happens if the process goes wrong are important questions, answers to which can have implications for many of the aberrant situations cells face, such as stress, aging and disease,” said Niwa.
Previously researchers in Niwa’s lab showed that a daughter cell can only viably separate if it receives a functional ER, thus completing a full round of cell division. Specifically, they found that when a mother cell does not have a sufficient level of ER for its own health or survival—a condition referred to as “ER stress”—the mother cell ceases giving ER to the daughter cell. At this point the mother cell halts the process of giving birth to a detached daughter cell.

Biological Sciences Professor Maho Niwa and Assistant Project Scientist Francisco Piña.
This finding led to the critical discovery of a previously unknown cell cycle checkpoint pathway that controls these events, which the researchers named the ER stress surveillance or “ERSU pathway.” This further unlocked several important questions. How does a cell recognize that its ER function is enough—or not enough—to continue generating a daughter cell? When the ER is stressed, how is ER inheritance stopped? And, when the ER is no longer stressed, how does the cell continue to generate a new daughter cell?
In the new study, Niwa and her colleagues provide some answers. Unexpectedly, they found that during ER stress the “septin ring,” one of the most important structural components involved in the growth and division of a daughter cell, moves away from the site of division, back into the mother and binds to a “birth mark” or “bud scar” (scientifically known as cytokinetic remnants) on the mother cell. This bud scar, marking a previous daughter’s birth mark, is prevented from being re-used for the birth of a new daughter cell specifically by a guardian protein called Nba1.
“We found that during ER stress, Nba1 effectively becomes a ‘midwife,’ moving components, including the septin ring—which manages the separation of the mother and daughter cells—back into the mother for safe keeping on the nearest bud scar,” said Niwa. “This results in a halt to the daughter’s growth and division is left for future use ‘on a sunny day.’”
If these components are not moved to the bud scar, the mother cell loses the ability to generate a new daughter when ER function is recovered. The researchers also found that these components “hop” along the bud scars from older to younger until they reach the youngest scar, similar to a frog on lily pads, Niwa said.
Probing further to discover what’s behind these changes, the researchers identified two sources: Slt2 kinase and Bem1, a scaffolding bud emergence protein. During ER stress, Slt2 is activated and Slt2/Bem1 move to the bud scar to cause the key Nba1 personality change.
Interestingly, Slt2 belongs to an evolutionally conserved family of proteins called MAP kinases, which respond to a wide array of stimuli, such as heat stress, osmotic stress, inflammation and growth stimuli. Accordingly, gaining an understanding of the ERSU cell cycle checkpoint pathway should help scientists understand how cells cope with other types of cellular catastrophes in the ER, including cancer and neuronal diseases such as Alzheimer’s.
The research was coauthored by Jesse Chao, Francisco Piña and Ya-Shiuan Lai of the Section of Molecular Biology, Division of Biological Sciences, UC San Diego; Masayuki Onishi of Stanford University School of Medicine; and Yifat Cohen and Maya Schuldiner of the Weizmann Institute of Science.
The research was supported the National Institutes of Health (R01GM087415), UC San Diego Chancellor’s Excellence Scholarship (formally FISP), the UC San Diego Academic Senate; the Volkswagen Foundation ‘‘Life’’ grant, The Deutsche Forschungsgemeinschaft (DFG) grant (SFB1190); a UC San Diego Molecular Biology Cancer postdoctoral fellowship; and an NIH 5T32AI007469-20UCSD/LIAI) allergy postdoctoral training grant.
