Jing Wang
Research
The olfactory sense is essential for basic animal behaviors such as finding food, avoiding predators, and locating mating partners. Understanding the mechanism of olfaction has been accelerated by the discovery of an olfactory map at the first synapse. In mammals, these synapses are organized into 1000 glomeruli, each of which receives thousands of sensory neurons that express the same odorant receptor gene. The driving force of my lab is to find answers to such questions as: Is the pattern of glomerular activity evoked by a given odor related to the detection of this odor? How is the olfactory information represented and processed in the brain? How is olfactory valence represented in the brain? How is this representation modulated to meet the demands of different internal physiological states?
The long-term goal of my laboratory is to elucidate the mechanism of olfaction. We choose Drosophila as a model system for two main reasons. First, Drosophila has fewer than 50 glomeruli in the antennal lobe. A sophisticated genetic toolkit makes it possible to establish causality between neural circuit function and behavior. Second, despite the numerical simplicity, the anatomical organization of the Drosophila olfactory system is fundamentally similar to that of mammals, suggesting a shared mechanism for smell. Our current research activities aim to identify specific neural circuits underlying olfactory behaviors, investigate how different physiological states of an organism modulate the function of olfactory circuits, and establish causality between neural plasticity in specific neuron populations and olfactory behaviors.
1) Synaptic modulations in neural circuits
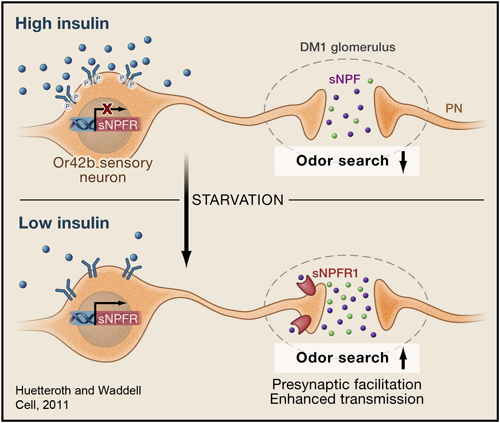
Olfaction is an ancient sense that makes important contributions to the perception of food quality and dietary selection. Learning how olfactory neural circuits impact dietary choices is relevant towards better understanding factors that contribute to obesity as well as anorexia in the infirm and elderly. Like many other animals, fruit flies exhibit different food searching and food-intake behaviors upon starvation. The sense of smell plays an important role in appetitive behavior in Drosophila. The DM1 glomerulus mediates attraction behavior and the DM5 glomerulus mediates avoidance behavior (Semmelhack and Wang, 2009). In a recent study, we showed that starvation sensitizes DM1's olfactory response (Root et al., 2011). A local signal by the neuropeptide sNPF/NPY and a global metabolic cue by insulin are integrated at specific sensory neurons to change olfactory sensitivity. This suggests the odor map is fine-tuned by the internal state of the animal.
Furthermore, our studies in the last few years have provided insights into establishing the notion that neuromodulation mechanisms are shared between Drosophila and mammals (Root et al., 2008; Dacks et al., 2009). In both groups, GABAB receptor-mediated presynaptic inhibition provides a gain control mechanism to modulate olfactory sensitivity. Furthermore, serotonin fine-tunes this feedback inhibition by controlling the excitability of the local interneurons in Drosophila and periglomerular cells in mice.
2) Neural plasticity in olfactory circuit
During a sensitive period in many animal species, usually early in life, sensory cues signaling safety and food are quickly learned and encoded into the memories of young animals through modifications of neurons in sensory neural circuits. As an important survival mechanism and a rudimentary form of learning, imprinting of sensory cues can guide and shape an animal's preferences and behaviors. We have obtained results to demonstrate that Drosophila exhibits a robust form of olfactory imprinting. Exposure to a given odorant in early adulthood dramatically increases a fly's preference for that odorant at a later stage. We aim to establish a causal link between neural plasticity in specific neuron populations and the imprinting behavior.By integrating several neural techniques, including single-neuron electrophysiology, optical imaging with genetically encoded activity indicators and genetic tools to silence or activate specific neurons in the stereotypic olfactory circuit, we hope to understand the neuronal bases of olfactory behaviors and test different hypotheses of olfactory codes with unprecedented resolution.
Select Publications
- Kim SM, Su CY, Wang JW. (2017) Neuromodulation of Innate Behaviors in Drosophila. Annu Rev Neurosci. 40:327-348.
- Sethi S and Wang JW. (2017) A versatile genetic tool for post-translational control of gene expression in Drosophila melanogaster. eLife. 6:e30327.
- Tao X, Lin HH, Lam T, Rodriguez R, Wang JW, Kubby J. (2017) Transcutical imaging with cellular and subcellular resolution. Biomedical Optics Express. 8(3):1277.
- Lin HH, Cao DS, Sethi S, Zeng Z, Chin JS, Chakraborty TS, Shepherd AK, Nguyen CA, Yew JY, Su CY, Wang JW. (2016) Hormonal Modulation of Pheromone Detection Enhances Male Courtship Success. Neuron. 90(6):1272-85.
- Ko, K.I., Root, C.M., Lindsay, S.A., Zaninovich, O.A., Shepherd, A.W., Wasserman, S.A., Kim, S.M., Wang, J.W. (2015) Starvation Promotes Concerted Modulation of Appetitive Olfactory Behavior via Parallel Neuromodulatory Circuits. eLife. 4:e08298.
- Masuyama K, Zhang Y, Rao Y, Wang JW. (2012) Mapping neural circuits with activity-dependent nuclear import of a transcription factor. J. Neurogenetics. 26: 89-102.
- Root C.M., Ko K.I., Jafari A., and Wang J.W. (2011) Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 145:133-144.
- Dacks A.M., Green D.S., Root C.M., Nighorn A.J., Wang J.W. (2009) Serotonin modulates olfactory processing in the antennal lobe of Drosophila. J. Neurogenetics 23:366-377.
- Semmelhack J.L. and Wang J.W. (2009) Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature 459:218-223.
- Root, C.M., Masuyama, K., Green, D.S., Enell, L.E., Nässel, D.R., Lee, C.H., Wang, J.W. (2008) A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron 59:311-321.
- Root, C.M., Semmelhack, J.L., Wong, A.M., Flores, J., Wang, J.W. (2007) Propagation of olfactory information in Drosophila. Proc. Natl. Acad. Sci. U S A. 104:11826-11831.
- Wang, J.W., Wong, A.M., Flores, J., Vosshall, L.B., and Axel, R. (2003). Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112:271-282.
- Wong, A.M., Wang, J.W., and Axel, R. (2002). Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109: 229-241.
Biography
Dr. Wang received his Ph.D. in Neurobiology from the University of Iowa. He did postdoctoral research in the laboratory of Alan Gelperin at Bell Labs, then in the laboratory of Richard Axel at Columbia University. Since joining the faculty in 2004, he has been named a Chris and Warren Hellman Faculty Scholar, a Searle Scholar, and a Beckman Young Investigator.

