James W. Golden
Research
Genetics, Molecular Biology, and Biotechnology of Cyanobacteria
Current areas of research include the development of improved genetic tools for cyanobacterial genetic engineering and the use of these tools to answer basic-science questions and for biotechnology applications. Our biotechnology-related research involves genetic engineering of cyanobacteria to synthesize bioactive natural products and biofuels. Other projects include identifying cyanobacterial genes related to resistance to grazing by amoebal and ciliate predators, the study of genes required for biosynthesis of cyanobacterial toxins, and the identification of cyanobacterial genes that affect fitness for growth during spaceflight or on Mars. These research projects include work with different strains of cyanobacteria, but focus on the laboratory model strain Synechococcus elongatus, strain PCC 7942, which has excellent genetics and is widely used for synthetic biology studies.
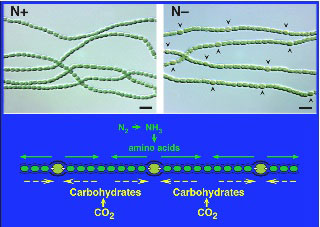
Past research has focused on the developmental biology of cyanobacterial heterocyst formation, with an emphasis on the genetic regulation of cellular differentiation and the cell-to-cell signaling mechanisms that control multicellular pattern formation. This research uses methods of genetics and molecular biology to understand basic principles of regulation and signaling pathways that control development in a simple prokaryotic multicellular organism, the filamentous cyanobacterium Anabaena (Nostoc), strain PCC 7120. Like all cyanobacteria, Anabaena uses light energy for photosynthesis. Anabaena is also capable of nitrogen fixation, a process that is incompatible with photosynthesis because the nitrogenase enzyme is destroyed by oxygen, a byproduct of photosynthesis. Anabaena solves this problem by spatially separating the two processes into different cell types: photosynthetic vegetative cells and nitrogen-fixing heterocysts. Anabaena grows as a very simple multicellular organism composed of filaments of vegetative cells containing about 10 percent heterocysts. Heterocysts differentiate from vegetative cells at semiregular intervals along the filament and supply fixed nitrogen to neighboring vegetative cells to support their growth. We identified a gene, patS, which encodes a small peptide that functions as a diffusible cell-to-cell signal that acts to control heterocyst pattern formation.

Select Publications
- Bishé B, Golden SS, Golden JW, Glycogen metabolism is required for optimal cyanobacterial growth in the rapid light-dark cycle of low-Earth orbit. Life Sciences in Space Research. 2023 36:18-26.
- Taton A, Rohrer S, Diaz B, Reher R, Caraballo-Rodriguez AM, Pierce ML, Dorrestein PC, Gerwick L, Gerwick WH, Golden JW. Heterologous expression in Anabaena of the columbamide pathway from the cyanobacterium Moorena bouillonii and production of new analogs. ACS Chemical Biology. 2022 17:1910-1923.
- Taton A, Ecker A, Diaz B, Moss NA, Anderson B, Reher R, Leão TF, Simkovsky R, Dorrestein PC, Gerwick L, Gerwick WH, Golden JW. Heterologous Expression of Cryptomaldamide in a Cyanobacterial Host. ACS Synth Biol. 2020 Dec 18;9(12):3364-3376. doi: 10.1021/acssynbio.0c00431. PMID: 33180461.
- Taton A, Erikson C, Yang Y, Rubin BE, Rifkin SA, Golden JW, Golden SS. The circadian clock and darkness control natural competence in cyanobacteria. Nat Commun. 2020 Apr 3;11(1):1688. doi: 10.1038/s41467-020-15384-9. PMID: 32245943; PMCID: PMC7125226.
- Kim Y-S, Park S-I, Kim J-J, Boyd JS, Beld J, Taton A, Lee K-I, Kim I-S, Golden JW and Yoon H-S (2020) Expression of Heterologous OsDHAR Gene Improves Glutathione (GSH)-Dependent Antioxidant System and Maintenance of Cellular Redox Status in Synechococcus elongatus PCC 7942. Front. Plant Sci. 11:231. doi: 10.3389/fpls.2020.00231.
- Bishé B, Taton A, Golden JW. 2019. Modification of RSF1010-Based Broad-Host-Range Plasmids for Improved Conjugation and Cyanobacterial Bioprospecting. iScience 20:216–228.
- Kumar K, Ota M, Taton A, Golden JW. 2019. Excision of the 59-kb fdxN DNA element is required for transcription of the nifD gene in Anabaena PCC 7120 Heterocysts. NZ J Bot 57:76–92.
- Taton A, Ma AT, Ota M, Golden SS, Golden JW. 2017. NOT Gate Genetic Circuits to Control Gene Expression in Cyanobacteria. ACS Synth Biol doi:10.1021/acssynbio.7b00203. pmcid:
- Chen, Y., A. Taton, M. Go, R. E. London, L. M. Pieper, S. S. Golden, and J. W. Golden. 2016. Self-replicating shuttle vectors based on pANS, a small endogenous plasmid of the unicellular cyanobacterium Synechococcus elongatus PCC 7942. Microbiology 162(12):2029-2041. PMID: 27902432.
- Taton, A., F. Unglaub, N. E. Wright, W. Y. Zeng, J. Paz-Yepes, B. Brahamsha, B. Palenik, T. C. Peterson, F. Haerizadeh, S. S. Golden, and J. W. Golden. 2014. Broad-host-range vector system for synthetic biology and biotechnology in cyanobacteria. Nucleic Acids Res. 42:e136. PMID: 25074377, PMCID: PMC4176158.
- Ma, A. T., C. M. Schmidt, and J. W. Golden. 2014. Regulation of gene expression in diverse cyanobacterial species by using theophylline-responsive riboswitches. Appl. Environ. Microbiol. 80:6704-6713. PMID: 25149516, PMCID: PMC4249034.
- Taton, A., E. Lis, D. M. Adin, G. Dong, S. Cookson, S. A. Kay, S. S. Golden, and J. W. Golden. 2012. Gene transfer in Leptolyngbya sp. strain BL0902, a cyanobacterium
Biography
Dr. James W. Golden received a B.S. (1977) in Microbiology from the University of Maryland-College Park and a Ph.D. (1983) in Biology from the University of Missouri-Columbia. After postdoctoral work as an NIH Fellow at The University of Chicago, he joined the Department of Biology at Texas A&M University in 1986. He was promoted to Associate Professor in 1990 and then to Professor in 1996. Dr. Golden moved to the University of California, San Diego in 2008.

