Researchers Examine How Cells Change the Pace of Their Steps
AUGUST 8, 2007
By Rex Graham
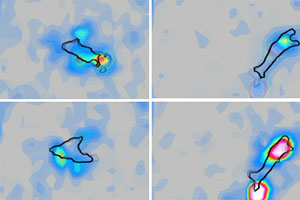
Chemotactic Motility of Amoeboid Cells
Scientists at UC San Diego have discovered how cells of higher organisms change the speed at which they move, a basic biological discovery that may help researchers devise ways to prevent cancer cells from spreading throughout the body.
The discovery reported by the UCSD scientists in Proceedings of the National Academy of Sciences (PNAS) and published Aug. 3 on the journal's Web site describes forces and energy exerted by the cells as they traveled across an elastic substrate. In videos recorded as the cells moved, each looked like an irregularly shaped water balloon attached firmly on two sticky sections while periodically protruding in the forward direction and withdrawing from the trailing end.
In humans and other mammals, cell motility is essential for many physiological processes such as tissue renewal and the function of the immune system. Cell motility also is an essential part of embryonic development as fetal cells undergo an orchestrated migration to form functioning tissues and organs. Poorly regulated cell motility during embryonic development may result in some neurological diseases and birth defects such as cleft palate of the mouth. UCSD's new findings may eventually be used to better understand and possibly treat such conditions and suggest possible new cancer treatments aimed at inhibiting the metastatic spreading of some cancer tumors.
Cells of all higher, or eukaryotic, species move in response to external stimuli. This movement is made possible by a series of inter-related biochemical reactions, some of which remodel the internal skeleton and others that add and remove adhesion points at strategic positions on the outer membrane. Regardless of their size or shape, cells use what cell biologists call the cell motility cycle to take one step per cycle: first, the cell extends its leading margin over the substratum forming a pseudopod or "foot-like" extension; secondly, the tip of the pseudopod develops an adhesion point that attaches to the substratum; next, the cell uses the new point of anchorage to contract; and finally, the trailing adhesion point detaches and the rear part of the cell retracts forward. The process repeats every 1 to 4 minutes in Dictyostelium cells, but the period of the cycle and the length of each step can be shorter or longer in other types of eukaryotic cells.
The scientists discovered that the crawling speed of Dictyostelium is not controlled by the sticking strength of the adhesion points, but rather by the frequency of the cell's motility cycle or how often they take a new step.
"For the first time, we've been able to make precise measurements of the repetitive nature of the forces and strain energies exerted by cells, and this has allowed us to better characterize the mechanics of the cell motility cycle," said Juan C. Lasheras, a co-author of the study and a professor of mechanical and aerospace engineering at UCSD's Jacobs School of Engineering.
A cell can assume a variety of shapes due to its internal "cytoskeleton," a network of crisscrossed protein fibers that forms an internal skeleton in a cell. The cytoskeleton is also involved in cell motility through its attachments to discrete adhesion regions on the cell membrane. As the cell moves, individual fibers can elongate at one end and shorten at the other.
"What has been lacking in the field is the ability to effectively measure and quantify the mechanical forces that cells use to move," said Richard Firtel, a professor of cell and developmental biology in UCSD's Division of Biological Sciences who co-directed the motility study with Lasheras. "Our study not only makes a major advance in understanding this key concept in biology, but also provides new tools that will allow us to make even more significant advances in the future. By using a model experimental cell such as Dictyostelium, we are able to design mutant strains that will permit us to dissect each component of the cell movement cycle, thus allowing us to understand the function of each biochemical part and how the whole system works in concert."
As the Dictyostelium cells moved on the elastic substrate the researchers measured strain forces of as much as 1,000 times greater than the weight of the cell. "These forces are truly amazing," said Lasheras. "It's comparable to a 200-pound athlete repeatedly lifting a 200,000-pound barbell. These Dictyostelium cells constantly maintain their cytoskeleton under this strong tension, although they periodically increase and decrease the strength as part of the motility cycle. The faster cells could repeat the cycle, the faster they moved."
The UCSD researchers examined a mutant strain of Dictyostelium that lacked an important cell adhesion protein called Talin, and to their surprise found that cells without Talin moved nearly as fast as wild-type cells. The finding suggests that the rate at which a cell can tighten and relax its cytoskeleton is more important in controlling its speed than how firmly it attaches to the substrate.
"Different cell types in the body can move at different speeds in response to many different stimuli, and while our collaborative study didn't look at these possibilities per se, we were able for the first time to correlate the mechanical forces related to chemical changes occurring within cells," said Firtel. "This study will help us understand the basis of a number of human genetics diseases and developmental abnormalities."
The co-authors include Firtel, Lasheras, Fulbright fellow Juan C. del Alamo, research scientist Ruedi Meili, Ph.D. candidate Baldomero Alonso-Latorre and postdoctoral fellows Javier Rodriguez-Rodriguez and Alberto Aliseda. The research was supported by a grant from the National Institutes of Health.
Related Links:
The Richard Firtel Lab
Media Contacts:
Rex Graham, (858) 822-3075
Kim McDonald, (858) 534-7572
