Alisa Huffaker
Research
Like animals, plants continually interact with a plethora of other organisms in their environment, including potentially pathogenic microbes and herbivorous insects that can cause disease or damage. To minimize the detrimental impact of invading organisms, plants must mount a rapid and robust immune response. My research interests center on plant peptide signals, Peps, which regulate broad spectrum plant defense responses against pathogens and herbivores, and their application to manipulate plant resistance to biotic attack.
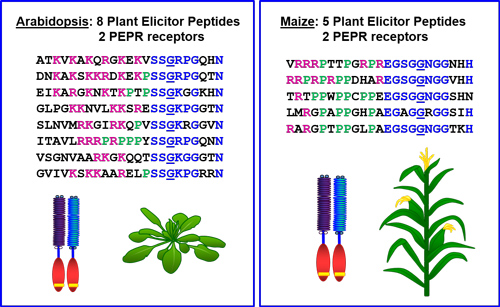
Multigene families encode Plant Elicitor Peptides (Peps) and their receptors (PEPRs), which act together to coordinate plant immunity.
Plant recognition of invading organisms occurs through perception of foreign molecules associated with attacking microbes and herbivores, known as elicitors, MAMPs (microbe-associated molecular patterns) or HAMPs (herbivore-associated molecular patterns). Perception of these molecules by pattern recognition receptors (PRRs) leads to subsequent launching of complex and multilayered defenses. These defenses, also known as basal or innate immune responses, have damaging effects on a wide array of pathogens and pests and involve accumulation of antimicrobial proteins and toxic metabolites. In addition to perceiving molecules from foreign organisms to detect biotic attack, plants also produce and recognize endogenous elicitors to assist in recognition and amplification of the immune response.
Arabidopsis Plant Elicitor Peptide1 (AtPep1) was the first endogenous peptide signal found to regulate plant anti-microbial defenses. AtPep1 is derived from a larger precursor encoded by the Arabidopsis gene designated AtPROPEP1, which is expressed in response to treatment with microbe-derived elicitors. AtPROPEP1 is a member of an eight gene family in Arabidopsis thaliana, each of which encode peptides that interact with the Plant Elicitor Peptide Receptors (PEPRs) to regulate plant immune responses. Treatment of Arabidopsis plants with AtPep1 induces production of defensive phytohormones and expression of pathogen defense genes. Transgenic over-expression of AtPROPEP1 causes constitutive expression of defense genes and enhances resistance to root degradation by the oomycete Pythium irregulare. Similarly, pretreatment of Arabidopsis plants with AtPeps prior to infection with Pseudomonas syringae Pv. tomato DC3000 restricts bacterial growth and decreases symptoms of disease.
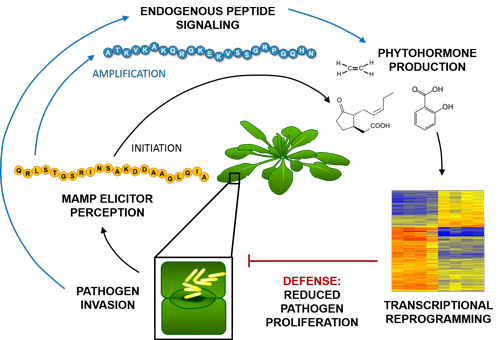
Peps coordinate and augment pathogen defense responses both spatially and temporally through amplification of MAMP-induced signaling.
AtPeps are active only in Arabidopsis and closely related Brassicaceous species, but gene orthologs have been identified in more than 50 plant species. These Pep orthologs also regulate innate immunity. Treatment of maize plants with the ortholog ZmPep1 prior to infection with fungal pathogens caused accumulation of defense transcripts and metabolites and reduced symptoms and cell death caused by both Cochliobolus heterostrophus (Southern leaf blight) and Colletotrichum heterostrophus (Anthracnose stalk rot).
Peps also mediate anti-herbivore defenses. Upon attack by herbivores, plants emit a complex blend of chemicals composed predominately of terpenes and six carbon (C6) green leafy volatiles (GLVs). These volatiles deployed by the plant recruit other organisms from the community to defend it from damage. Beneficial insects such as wasp species are attracted to the volatile blend and either eat or lay eggs in the attacking herbivorous pests. A maize Plant Elicitor Peptide, ZmPep3, is a potent signal promoting emission of herbivore associated volatiles and treatment of maize leaves with ZmPep3 results in accumulation of transcripts encoding biosynthetic enzymes for volatiles and other defense metabolites. These ZmPep3-induced responses are effective as direct and indirect defenses against herbivores, limiting growth of Spodoptera exigua larvae and attracting naïve Cotesia marginiventris, a parasitoid of Lepidopteran larvae. Similar regulation of anti-herbivore defense by Peps occurs in numerous and diverse plant species.
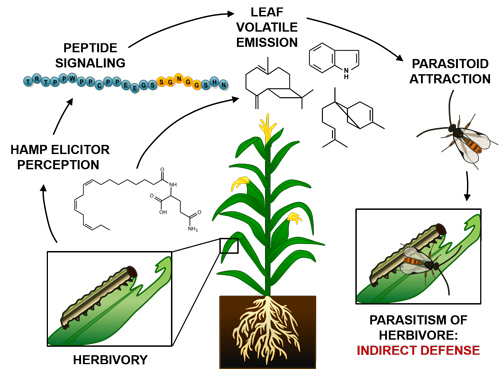
Some Peps promote plant defense against herbivores.
Because PROPEP genes and signaling by Peps are ubiquitous throughout angiosperm plants, knowledge gleaned from Pep research in maize and Arabidopsis can be extrapolated and applied to other plants. Peps act as regulatory nodes for defenses against pathogens and herbivores, and are valuable candidate tools for genetic improvement of crop resistance. Study of Peps have generated a number of compelling questions for future research. How is signal specificity achieved? What signaling components are involved? How can this endogenous regulation of the plant immune system be improved to protect plants?
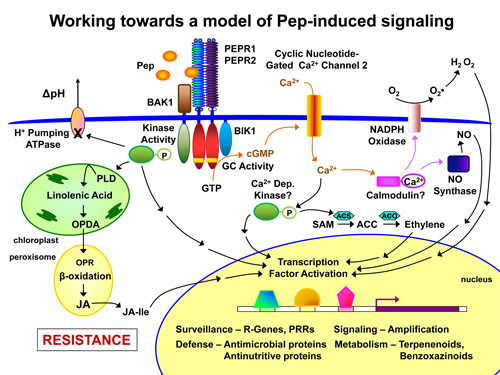
As individual Peps appear to have differing functions, we’re studying the overlap of signaling and defense strategies mediated by individual Peps and by exogenous elicitors from other organisms. We hope to decode Pep-induced response specificity and to identify molecular components that can be successfully used to enhance resistance against a variety of attackers, including biotrophic and nectrotrophic pathogens as well as specialist and generalist herbivores. Current lab efforts are focused on 1. Study of Pep-PEPR ligand-receptor interactions and determination of structural requirements for signaling activity. 2. Characterization of downstream Pep signaling components identified through genetic screens and profiling of rapid changes in transcriptome and phosphoproteome of maize and Arabidopsis. 3. Development of Pep-based strategies for enhancement of plant immunity against pathogens and pests.
Go to full publication list
Select Publications
- Schmelz EA, Huffaker A, Sims JW, Christensen SA, Lu X, Okada K, Peters RJ (2014) Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 79(4):659-78. PMID: 24450747
- Huffaker A, Pearce G, Veyrat N, Erb M, Turlings TC, Sartor R, Shen Z, Briggs SP, Vaughan MM, Alborn HT, Teal PE, Schmelz EA (2013) Plant elicitor peptides are conserved signals regulating direct and indirect antiherbivore defense. Proc Natl Acad Sci U S A. 110(14):5707-12. PMID: 23509266
- Schmelz EA, Huffaker A, Carroll MJ, Alborn HT, Ali JG, Teal PE (2012) An amino acid substitution inhibits specialist herbivore production of an antagonist effector and recovers insect-induced plant defenses. Plant Physiol. 160(3):1468-78. PMID: 23008466
- Anderson RG, Casady MS, Fee RA, Vaughan MM, Deb D, Fedkenheuer K, Huffaker A, Schmelz EA, Tyler BM, McDowell JM (2012) Homologous RXLR effectors from Hyaloperonospora arabidopsidis and Phytophthora sojae suppress immunity in distantly related plants. Plant J. doi: 10.1111/j.1365-313X.2012.05079.x. PMID: 22709376
- Dafoe NJ, Huffaker A, Vaughan MM, Duehl AJ, Teal PE, Schmelz EA (2011) Rapidly induced chemical defenses in maize stems and their effects on short-term growth of Ostrinia nubilalis. J Chem Ecol. 37(9):984-91. PMID: 21833765
- Huffaker A, Kaplan F, Vaughan MM, Dafoe NJ, Ni X, Rocca JR, Alborn HT, Teal PE, Schmelz EA (2011) Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize. Plant Physiol. 156(4):2082-97. PMID: 21690302
- Yamaguchi Y, Huffaker A (2011) Endogenous peptide elicitors in higher plants. Curr Opin Plant Biol. 14(4):351-7. PMID: 21636314
- Schmelz EA, Kaplan F, Huffaker A, Dafoe NJ, Vaughan MM, Ni X, Rocca JR, Alborn HT, Teal PE (2011) Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc Natl Acad Sci U S A. 108(13):5455-60. PMID: 21402917
- Huffaker A, Dafoe NJ, Schmelz EA (2011) ZmPep1, an ortholog of Arabidopsis elicitor peptide 1, regulates maize innate immunity and enhances disease resistance. Plant Physiol. 155(3):1325-38. PMID: 21205619
- Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA (2010) PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell. 22(2):508-22. PMID: 20179141
- Ryan CA, Huffaker A, Yamaguchi Y (2007) New insights into innate immunity in Arabidopsis. Cell Microbiol. 9(8):1902-8. PMID: 17593247
- Huffaker A, Ryan CA (2007) Endogenous peptide defense signals in Arabidopsis differentially amplify signaling for the innate immune response. Proc Natl Acad Sci U S A. 104(25):10732-6. PMID: 17566109
Biography
Dr. Huffaker received her Ph.D. from the Institute of Biological Chemistry at Washington State University. She continued at the Institute of Biological Chemistry for her postdoctoral training in plant defense signaling. Dr. Huffaker joined the section of Cell and Developmental Biology in 2014.

