Eric Schmelz
In Memoriam: Professor Eric Schmelz, Cell and Developmental BiologyResearch
I have always been fascinated by the existence of specific biochemicals that significantly govern interactions between organisms. Plants, arthropods and microbes are the most species rich life forms on earth. As primary producers, photosynthetic organisms are the ultimate source of most fixed carbon and under significant pressure to relinquish these resources. For reproductive success, plants must balance both growth and defense against a myriad of herbivorous arthropods and pathogens. These real-time interactions are of relevance to our global food supply and are underpinned by genetics, biochemistry, physiology, and behavior.
We utilize maize ( Zea mays) and bean ( Phaseolus & Vigna spp.) to examine dynamic inducible innate immune responses that limit losses against attacking organisms. In response to herbivory by generalist lepidopteran pests, plants rapidly initiate the biosynthesis and release of low molecular weight Volatile Organic Compounds (VOCs). Prominent in this VOC signature are monoterpenes, homoterpenes and sesquiterpenes that serve as indirect plant defenses by enabling the attraction of predators, parasitoids, and other natural enemies. How is this process enabled? We examine the mechanistic role of exogenous elicitors, termed Herbivore Associated Molecular Patterns (HAMPs), and endogenous signal transduction cascades that regulate insect-induced plant responses.
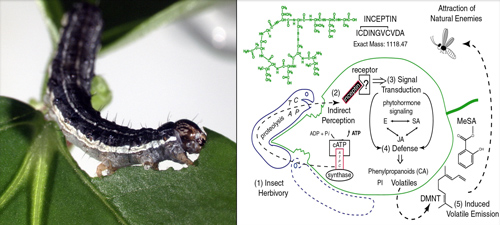
Plant recognition and elicitation of inducible volatiles following herbivory in beans ( Phaseolus and Vigna spp.) is mediated by femtomolar amounts of the peptide elicitor termed inceptin, derived from chloroplastic ATP synthase γ-subunit proteolysis. Specialist herbivores evade detection by converting inceptin into a potent antagonist of elicitation. Molecular level details of herbivore perception by plants are essential for understanding defense and susceptibility in crops.
In contrast to foliar herbivores, maize fungal pathogens elicit the biosynthesis of non-volatile acidic terpenoid phytoalexins with direct antibiotic activity. The most highly inducible transcripts following pathogen attack encode the β-macrocarpene synthases (ZmTps) 6/11 and the ent-copalyl diphosphate synthase termed Anther Ear 2 (ZmAn2). Down stream predicted enzymatic products of ZmTps6/11 and ZmAn2, termed zealexins and kauralexins respectively, are the predominant phytoalexins of mature maize plants. These recent discoveries raise many questions including: what exogenous and endogenous signals strongly promote maize phytoalexin biosynthesis? What are the roles of these terpenoids in plant protection? plant signaling? rhizosphere interactions? allelopathy? autotoxicity? and human health? Given that approximately 150,000 square of maize is annually planted in the U.S. and that fungal attack is aggressive during senescence it is of interest to know, where do these compounds go? what is their fate in the environment?

(A) Maize plants must defend against significant biotic threats that include fungal pathogens [(B) southern leaf blight, Cochliobolus heterostrophus; (C) stalk rot, Fusarium graminearum; (D) ear rot, Aspergillus flavus) and stem boring lepidoptera [(E) European corn borer: Ostrinia nubilalis]. All of these stimuli result in localized zealexin and kauralexin production to limit microbial spread.
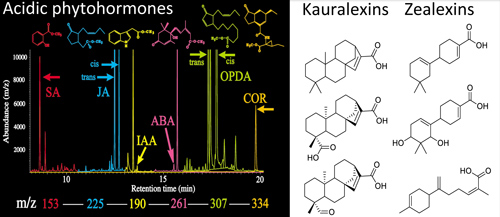
Mass spectrometry-based metabolomic profiling of phytohormones and defenses is a powerful tool to aid in the discovery of novel metabolites that additionally serve as signals and plant protectants.
We utilize biochemical discovery approaches based on the coupling of separations with rapid activity based bioassays, NMR structure elucidation, mass spectrometry for the plant metabolomic analyses of novel induced defenses and signals, genetic resources to test candidate pathways, global expression analyses for co-regulated processes, expression quantitative trait loci (eQTL) for gene discovery, transient and heterologous expression of enzymes for product analyses, and other tools to uncover previously hidden pathways and mechanisms underlying plant-mediated interactions with their biotic and abiotic environment.
Go to full publication list
Select Publications
- Christensen SA, Huffaker A, Kaplan F, Sims J, Ziemann S, Doehlemann G, Ji L, Schmitz RJ, Kolomiets MV, Alborn HT, Mori N, Jander G Ni X, Sartor RC, Byers S, Abdo Z, Schmelz EA (2015) Maize death acids, 9-lipoxygenase-derived cyclopente(a)nones, display activity as cytotoxic phytoalexins and transcriptional mediators. Proc. Natl. Acad. Sci. USA 112:11407-11412
- Christensen SA, Huffaker A, Hunter CT, Alborn HT, Schmelz EA (2015) A maize death acid, 10-oxo-11-phytoenoic acid, is the predominant cyclopentenone signal present during multiple stress and developmental conditions. Plant Signal Behav. 15:0 [Epub ahead of print]
- Yan J, Lipka AE, Schmelz EA, Buckler ES, Jander G (2015) Accumulation of 5-hydroxynorvaline in maize (Zea mays) leaves is induced by insect feeding and abiotic stress. J. Exp. Bot. 66(2): 593–602
- Vaughan MM, Christensen S, Schmelz EA, Huffaker A, McAuslane HJ, Alborn HT Romero M, Allen L H, and Teal PEA (2015) Accumulation of terpenoid phytoalexins in maize roots is associated with drought tolerance. Plant Cell Environ. 38(11): 2195–2207
- Schmelz EA (2015) Impacts of insect oral secretions on defoliation-induced plant defense. Current Opinion in Insect Science. 9:7–15
- Tzin V, Fernandez-Pozo N, Richter A, Schmelz EA, Schoettner M, Schäfer M, Ahern KR, Meihls LN, Kaur H, Huffaker A, Mori N, Degenhardt J, Mueller LA, Jander G (2015) Dynamic Maize Responses to Aphid Feeding Are Revealed by a Time Series of Transcriptomic and Metabolomic Assays. Plant Physiol. 169:1727-1743
- Martins V, Vaughan MM, Huffaker A, Schmelz EA, Christensen S, Sims J, Benda ND, Swerbilow J, Alborn H, Teal PE (2014) Seed treatment with live or dead Fusarium verticillioides equivalently reduces the severity of subsequent stalk rot. Journal of Phytopathology 162(3): 201–204
- Christensen S, Nemchenko A, Park Y-S, Schmelz E, Kunze S, Feussner I, Yalpani N, Meeley R, Kolomiets M (2014) The novel maize 9-lipoxygenase, ZmLOX12, is required to mount an effective defense against Fusarium verticillioides. Mol. Plant. Microbe Interact. 27(11): 1263-76.
- Vu HS, Roth MR, Tamura P, Samarakoon T, Shiva S, Honey S, Lowe K, Schmelz EA, Williams TD, Welti R (2014). Head-group acylation of monogalactosyldiacylglycerol is a common stress response, and the acyl-galactose acyl composition varies with the plant species and applied stress. Physiol. Plant. 150(4): 517–528.
- Schmelz EA, Huffaker A, Sims J, Christensen S, Okada K, Peters RJ (2014) Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 79(4): 659-678 Shen J, Tieman D, Jones JB, Taylor MG, Schmelz E, Huffaker A, Bies D, Chen K, Klee HJ (2014) A 13-lipoxygenase, TomloxC, is essential for synthesis of C5 flavour volatiles in tomato. J Exp. Bot. 65(2): 419-428
- Ni X, Toews MD, Buntin GD, Carpenter JE, Huffaker A, Schmelz EA, Cottrell TE, Abdo Z (2014) Influence of brown stink bug feeding, planting date and sampling time on common smut infection of maize. Insect Sci. 21(5): 564-571
- Vaughan MM, Huffaker A, Schmelz EA, Dafoe NJ, Christensen S, Sims J, Martins VF, Swerbilow J, Romero M, Alborn HT, Allen LH, Teal PE (2014) Effects of elevated [CO2] on maize defense against mycotoxigenic Fusarium verticillioides. Plant Cell Environ. 37(12): 2691–2706
- Murakami S, Nakata R, Aboshi T, Yoshinaga N, Teraishi M, Okumoto Y, Ishihara A, Morisaka H, Huffaker A, Schmelz EA, Mori N (2014) Insect-induced daidzein, formononetin and their conjugates in soybean leaves. Metabolites, 4(3): 532-546
- Dafoe NJ, Thomas JD, Shirk PD, Legaspi ME, Vaughan MM, Huffaker A, Teal PE, and Schmelz EA (2013) European corn borer (Ostrinia nubilalis) induced responses enhance susceptibility in maize. PLoS ONE 8(9): e73394
- Huffaker A, Pearce G, Veyrat N, Erb M, Turlings TCJ, Sartor R, Shen Z, Briggs SP, Vaughan MM, Alborn HT, Teal PEA, Schmelz, EA (2013) Plant Elicitor Peptides are Conserved Signals Regulating Direct and Indirect Anti-Herbivore Defense. Proc. Natl. Acad. Sci. USA 110:5707-12
- Anderson RG, Casady MS, Fee RA, Vaughan MM, Deb D, Fedkenheuer K, Huffaker A, Schmelz, EA, Tyler, BM and McDowell, JM (2012) Homologous RXLR effectors from Hyaloperonospora arabidopsidis and Phytophthora sojae suppress immunity in distantly related plants. Plant Journal 72, 882–893
- Schmelz EA, Huffaker A, Carroll MJ, Alborn HT, Ali JG, Teal PE (2012) An amino acid substitution inhibits specialist herbivore production of an antagonist effector and recovers insect-induced plant defenses. Plant Physiology 160: 1468-1478
- Dafoe NJ, Huffaker A, Vaughan MM, Duehl AJ, Teal PE, Schmelz EA (2011) Rapidly induced chemical defenses in maize stems and their effects on short-term growth of Ostrinia nubilalis. J. Chem. Ecol. 37:984-991
- Schmelz EA, Kaplan F, Huffaker A, Dafoe NJ, Vaughan MM, Ni X, Rocca JR, Alborn HT, Teal PEA (2011) Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc. Natl. Acad. Sci. USA 108: 5455-5460
- Huffaker A, Kaplan F, Vaughan MV, Dafoe NJ, Ni X, Rocca JR, Alborn HT, Teal PEA, Schmelz EA (2011) Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize. Plant Physiol. 156: 2082-2097
- Acosta IF, Laparra H, Romero SP, Schmelz E, Hamberg M, Mottinger JP, Moreno MA, Dellaporta SL (2009) "tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize" Science 323(5911): 262-265
- Schmelz EA, Engelberth J, Alborn HT, Tumlinson JH, Teal PEA (2009) Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc. Natl. Acad. Sci. USA 106: 653-657
- Schmelz EA, LeClere S, Carroll MJ, Alborn HT, Teal PEA (2007) Cowpea chloroplastic ATP synthase is the source of multiple plant defense elicitors during insect herbivory. Plant Physiol.144: 793-805
- Alborn HT, Hansen TV, Jones TH, Bennett DC, Tumlinson JH, Schmelz EA, Teal PEA (2007) Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc. Natl. Acad. Sci. USA 104: 12976-12981
- Schmelz EA, Carroll MJ, LeClere S, Phipps SM, Meredith J, Chourey PS, Alborn HT, Teal PEA (2006) Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. USA 103: 8894-8899
Biography
Eric Schmelz completed his PhD within the Center for Insect Science/Plant-Insect Interactions Group at the University of Arizona, Tucson. He then carried out his postdoctoral and research scientist studies at the USDA-Agricultural Research Service located at the University of Florida, Gainesville. He joined the Division of Biological Sciences faculty at U.C. San Diego in July 2014.

