Nabora Reyes de Barboza
Research
To date, cellular senescence is defined as an acquired state cells enter in response to environmental stressors or as a means of tumor evasion. Senescent cells cease their proliferative capacity and enhance their ability to respond and regulate microenvironments through the senescence-associated secretory phenotype (SASP). Another characteristic of cellular senescence is the upregulation of the cyclin inhibitor p16 INK4a . With age there is accumulation of senescent cells in tissues along with upregulation of p16 INK4a expression. While removal of p16 INK4a expressing cells (p16+) using genetic mouse models slows down aging, it also adversely impacts wound healing, suggesting contradictory roles of p16 INK4a during homeostasis, regeneration, and aging. Our understanding of the dynamic, and pleiotropic nature of the SASP in senescent cells remains limited. Therefore, further work is required to elucidate how the SASP alters tissue microenvironments, contributes to injury-specific cellular response and whether this process can be controlled to develop novel therapeutic approaches to lung injury.
Our team investigates the behaviors, characteristics, and consequences of secreted protein induction by senescent cells in vivo, with a particular focus on lung contexts. Our research aims to advance understanding and potential therapeutic applications by examining cellular responses within lung microenvironments following acute injury and repair. We employ advanced mouse genetic tools, physiology, cutting-edge sequencing technologies, and quantitative proteomics to explore the functions of senescent cells within region-specific lung microenvironments.
Research Questions
What roles do alveolar p16+ fibroblasts play in promoting repair of alveolar type 2 (AT2) cells?
We discovered that p16+ fibroblasts enhance airway epithelial regeneration both in vitro and in vivo. These findings strongly suggest that fibroblasts expressing p16 INK4a induce a SASP conducive to driving epithelial regeneration. However, it remains unclear whether this supportive quality is limited to fibroblasts surrounding major airways. Therefore, we are investigating the hypothesis that p16+ fibroblasts activate a region-specific regenerative program tailored to support local stem cells.
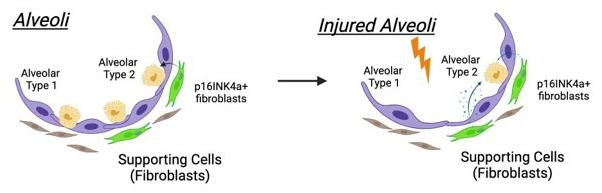
What is the role of p16 INK4a in reprogramming the fibroblast secretome?
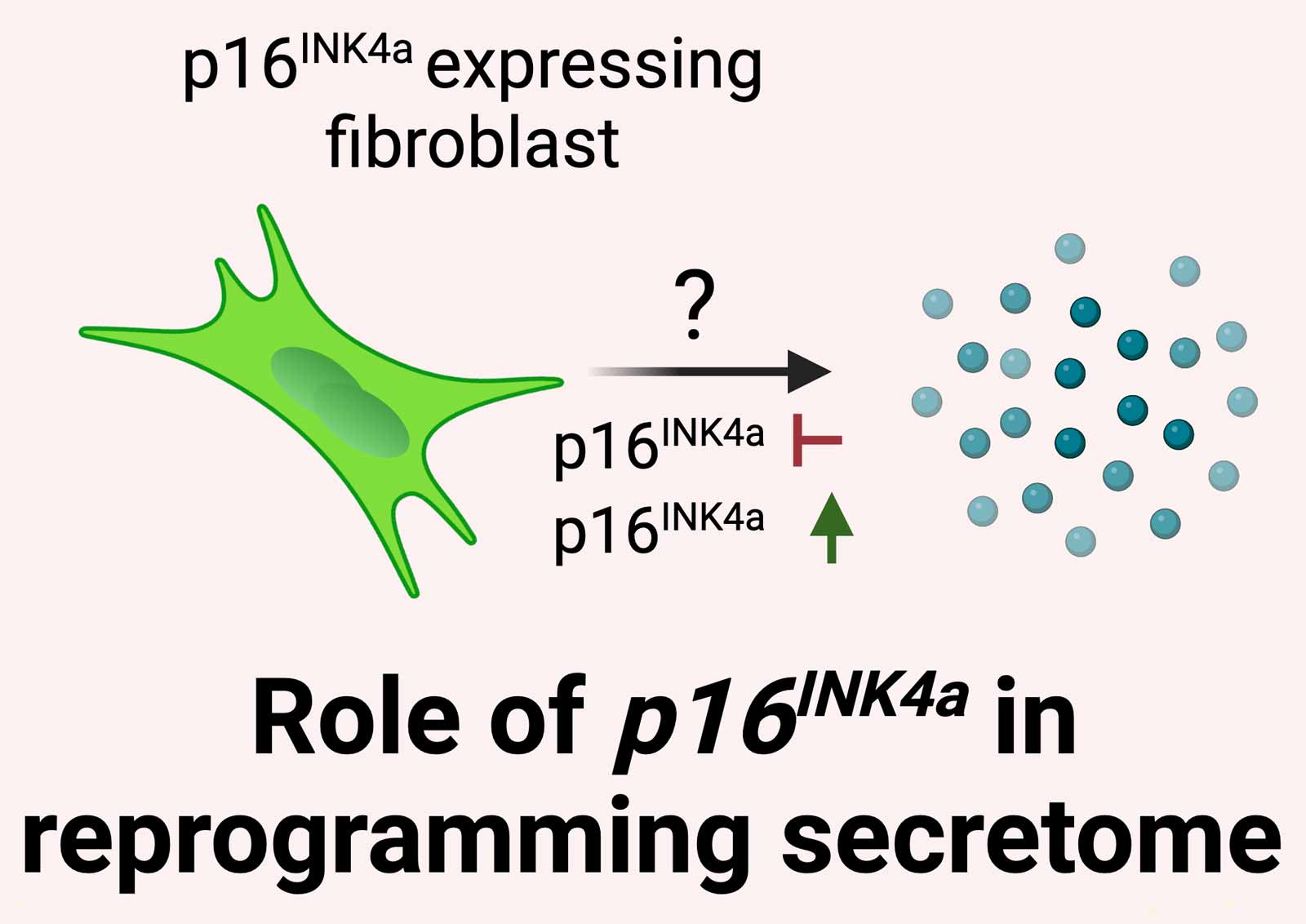
We have demonstrated that p16 INK4a expression is essential for inducing protein secretion in fibroblasts. By manipulating p16 INK4a expression using our established tools and combining transcriptomic and proteomic approaches, we aim to define p16 INK4a 's ability to reprogram the SASP in fibroblasts to promote regeneration in neighboring stem cells. Our goal is to determine whether protein secretion depends on p16 INK4a expression under homeostatic conditions and during injury.
Select Publications
- Lee JY, Reyes NS, Ravishankar S, Zhou M, Krasilnikov M, Ringler C, Pohan G, Wilson C, Ang KK, Wolters PJ, Tsukui T, Sheppard D, Arkin MR, Peng T. An in vivo screening platform identifies senolytic compounds that target p16INK4a+ fibroblasts in lung fibrosis. J Clin Invest. 2024 Mar 7;134(9). doi: 10.1172/JCI173371. PubMed PMID: 38451724; PubMed Central PMCID: PMC11060735.
- Chen X, Haribowo AG, Baik AH, Fossati A, Stevenson E, Chen YR, Reyes NS, Peng T, Matthay MA, Traglia M, Pico AR, Jarosz DF, Buchwalter A, Ghaemmaghami S, Swaney DL, Jain IH. In vivo protein turnover rates in varying oxygen tensions nominate MYBBP1A as a mediator of the hyperoxia response. Sci Adv. 2023 Dec 8;9(49):eadj4884. doi: 10.1126/sciadv.adj4884. Epub 2023 Dec 8. PubMed PMID: 38064566; PubMed Central PMCID: PMC10708181.
- Nabora S. Reyes, Maria Krasilnikov, Nancy Allen, Jin Young Lee, Ben Hyams, Mingi Zhou, Supriya Ravishankar, Monica Cassandras, Chaoqun Wang, Imran Khan, Peri, Matatia, Yoshikazu Johmura, Ari Molofsky, Michael Matthay, Makoto Nakanishi, Dean Sheppard, Judith Campisi, and Tien Peng. (2022) Sentinel p16INK4a cells in the basement membrane form a reparative niche in the lung. Science, 378:(6616), 192-201 DOI: 10.1126/science.abf3324.
- Nig Ma*, Nabora Reyes de Mochel*, Paula Duyen Pham, Tae Yeon Yoo, Ken W.Y. Cho and Michelle A. Digman. (2019) Label-free assessment of preimplantation embryo quality by the Fluorescence lifetime imaging microscopy (FLIM)-phasor approach. Scientific Reports. 9(1):13206 PMCID: PMC6744410 *Equally contributing authors, co-first authors of the work.*Work has a patent, UC Case No. 2018-517-1, reporting # 0577501-18-0026.
- Chaoqun Wang, Nabora S. Reyes de Mochel, Stephanie A. Christenson, Monica Cassandras, Rebecca Moon, Alexis N. Brumwell, Lauren E. Byrnes, Alfred Li, Yasuyuki Yokosaki, Peiying Shan, Julie B. Sneddon, David Jablons, Patty J. Lee, Michael A. Matthay, Harold A. Chapman, and Tien Peng. (2018) Expansion of hedgehog disrupts mesenchymal identity and induces emphysema phenotype. The Journal of Clinical Investigation 128(10):4343-4358. PMCID: PMC6159975.
- William R. Holmes*, Nabora Reyes de Mochel*, Qixuan Wang, Huiing Du, Michael Chiang, Olivier Cinquin, Ken W.Y. Cho and Qing Nie. (2017). Gene Expression Noise Enhances Robust Organization of the Early Mammalian Blastocyst. PLOS Computational Biology. PMCID: PMC5293272.*Equally contributing authors, co-first authors of the work.
- Michael Chiang, Sam Hallman, Amanda Cinquin, Nabora Reyes de Mochel, Adrian Paz, Shimako Kawauchi, Anne L. Calof, Ken W. Cho, Charles C. Fowlkes, and Olivier Cinquin. (2015) Analysis of in vivo single cell behavior by high throughput, human-in-the-loop segmentation of three-dimensional images. (BMC Bioinformatics May 2015).
- Nabora S. Reyes de Mochel, Mui Luong, Michael Chiang, Anna L. Javier, Elizabeth Luu, Fujimori Toshihiko, Grant R. MacGregor, Olivier Cinquin and Ken W.Y. Cho. (2015) BMP signaling regulates both cell proliferation and ICM lineage commitment in preimplantation-stage mouse embryos. Dev Bio 397(1):45-55.
- Anna L. Javier, Linda T. Doan, Mui Luong, N. Soledad Reyes de Mochel, Aixu Sun, Edwin S. Monuki, and Ken W. Y. Cho. (2012). Bmp indicator mice reveal dynamic regulation of transcriptional response. PLOS one, 7 (9): e42566,PMC3439458.
- Delphine Eberlé, Roy Y. Kim, Fu San Luk, N. Soledad Reyes de Mochel, Nathalie Gaudreault, Victor R. Olivas, Nikit Kumar, Jessica M. Posada, Andrew Birkeland, Joseph H. Rapp and Robert L. Raffai. (2012) Apolipoprotein E4 “domain interaction” accelerates diet-induced atherosclerosis in hypomorphic Arg-61 Apoe mice. ATVB, 32, 1116-1123, PMC3346714.
- Nathalie Gaudreault, Nikit Kumar, Jessica M. Posada, Kyle B. Stephens, N. Soledad Reyes de Mochel, Delphine Eberle, Victor Olivas, Roy Kim, Matthew J. Harms, Amy Johnson, Louis M. Messina, Joseph H. Rapp, and Robert L. Raffai. (2012). Apo E reduces atherosclerosis by suppressing lipid-induced leukocytosis and the activation of monocytes and vascular endothelium. ATVB, 32, 244-272.
- R-M Liu, J. Choi, J-H Wu, K. A. Gaston Pravia, K Lewis, J. D. Brand, N. S Reyes Mochel, M. Krywanski, J. D. Lambeth, J Hagood, H. J. Forman, V. J. Thannickal, and E. M. Postlethwait. (2010). Oxidative modification of nuclear mitogen activated protein kinase phosphatase 1 is involved in transforming growth factor beta1-induced expression of plasminogen activator inhibitor 1 in fibroblasts. JBC, 285, 16239-16247, PMC2871491.
- Nabora Soledad Reyes de Mochel, Scott Seronello, Chieri Ito, Jasper Xi Zheng, Jake Liang, J.R.David Lambeth, and Jinah Choi. (2010). Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology, 52(1), 47-59.
- Hui Zhu, N. Soledad Reyes, & Matthew P. Meyer. (2009). Computational and experimental structure-reactivity relationships: evidence for a side reaction in Alpine-Borane reductions of d-benzaldehydes. Tetrahedron Letters, 50, 6803-6806.
Biography
Nabora Reyes de Barboza earned her BS in Biochemistry and Molecular Biology and MS in Quantitative and Systems Biology from UC Merced. Her early career focused on signaling systems, studying Nox proteins' role in hepatitis C during her master's. For her PhD at UC Irvine with Dr. Ken Cho, she researched BMP's role in pre-implantation development. As a postdoctoral fellow at UCSF under Dr. Tien Peng, she investigated senescent cell signaling in lung regeneration. Her work earned prestigious awards, including the NRSA F32, UC Chancellor’s, and UCSF-CIRM Postdoctoral Fellowships and NHLBI K99/R00 Pathway to Independence Award. Dr. Reyes joined the faculty in Cell and Developmental Biology July 2024.

