Scott Biering
Research
Emerging and re-emerging viruses pose some of the greatest threats to human health. This threat is epitomized by the mosquito-transmitted flaviviruses such as dengue virus, with over half the world's population at risk of infection, and SARS-CoV-2, the causative agent of the devastating and ongoing COVID-19 pandemic. We need novel strategies to target multiple viruses given our current challenges in developing efficacious therapeutics and vaccines and our inability to predict future viral epidemics. To this end, our lab aims to identify and characterize general principles of viral pathogenesis common to multiple viruses to identify new targets for vaccines and therapeutics. We are particularly focused on host-virus interactions at the interface of physiological barriers like the endothelium or epithelium since virtually all viruses must traverse these barriers to establish a productive infection and/or transmit to subsequent hosts. Based on this commonality across viruses, we hypothesize that disrupting the capacity of viruses to break through these barriers is a viable strategy to block pathogenesis for a broad range of viruses. The research in our lab addresses this central hypothesis with projects falling into four aims:
- Define the mechanisms by which viruses disrupt host barriers.
- Investigate how barrier dysfunction impacts virus infection and pathogenesis.
- Explore host defense mechanisms that prevent virus-triggered barrier dysfunction.
- Develop therapeutic and vaccine strategies blocking virus-triggered barrier dysfunction.
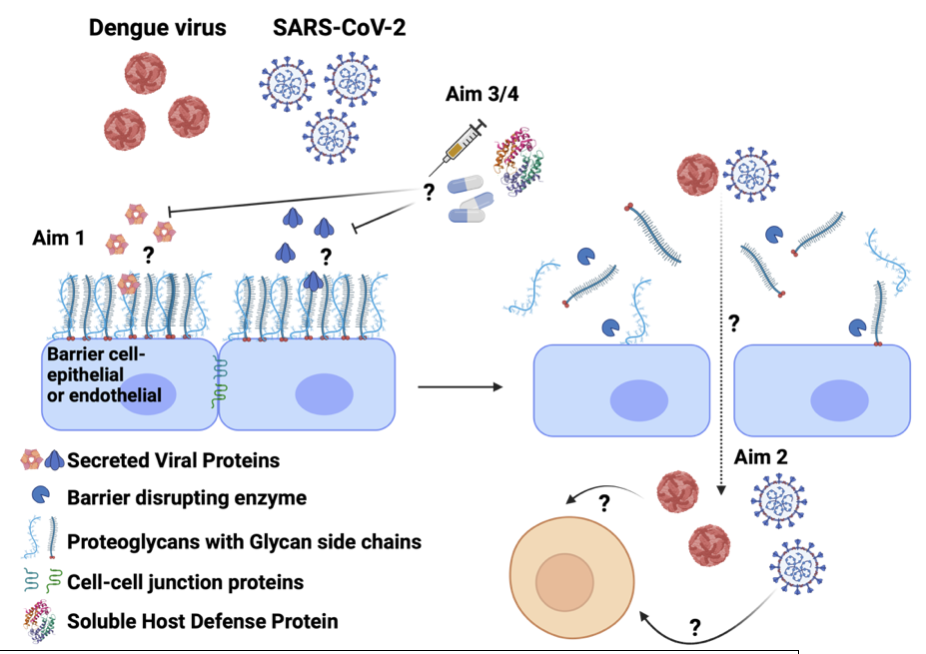
Viruses like dengue virus and SARS-CoV-2 use soluble viral proteins to break through cellular barriers. These viral proteins interact with barrier cells to activate signaling pathways that mediate barrier dysfunction (Aim 1). Viruses then traverse these barriers to gain access to new cellular targets of infection (Aim 2). Our hypothesis is that targeting the interactions between viral proteins and barrier cells is a viable strategy to block viral pathogenesis (Aim 3/4).
Select Publications
- Biering, S. B.*,#, Gomes de Sousa, F. T.*, Tjang, L. V., Pahmeier, F., Zhu, C., Ruan, R., Blanc, S. F., Patel, T. S., Worthington, C. M., Glasner, D. R., Castillo-Rojas, B., Servellita, V., Lo, N. T. N., Wong, M. P., Warnes, C. M., Sandoval, D. R., Clausen, T. M., Santos, Y. A., Fox, D. M., Ortega, C., Näär, A. M., Baric, R. S., Stanley, S. A., Aguilar, H. C., Esko, J. D., Chiu, C. Y., Pak, J. E., Beatty P. R., Harris, E#. (2022). SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-β signaling. Nature Communications, 13(1), 7630. https://doi.org/10.1038/s41467-022-34910-5 *equal contribution, #co-corresponding authors
- Lo, N. T. N., Roodsari, S. Z., Tin, N. L., Wong, M. P., Biering, S. B.#, & Harris, E#. (2022). Molecular Determinants of Tissue Specificity of Flavivirus Nonstructural Protein 1 Interaction with Endothelial Cells. Journal of Virology, 96(19), e0066122. https://doi.org/10.1128/jvi.00661-22 #co-corresponding authors
- Biering, S. B.*, Sarnik, S. A.*, Wang, E., Zengel, J. R., Leist, S. R., Schäfer, A., Sathyan, V., Hawkins, P., Okuda, K., Tau, C., Jangid, A. R., Duffy, C. V., Wei, J., Gilmore, R. C., Alfajaro, M. M., Strine, M. S., Nguyenla, X., Van Dis, E., Catamura, C., Yamashiro, L. H., Belk, J. A., Begeman, A., Stark, J. C., Shon, D. J., Fox, D. M., Ezzatpour, S., Huang, E., Olegario, N., Rustagi, A., Volmer, A. S., Livraghi-Butrico, A., Wehri, E., Behringer, R. R., Cheon, D. -J., Schaletzky, J., Aguilar, H. C., Puschnik, A. S., Button, B., Pinsky, B. A., Blish, C. A., Baric, R. S., O’Neal, W. K., Bertozzi, C. R., Wilen, C. B., Boucher, R. C., Carette, J. E., Stanley, S. A., Harris, E., Konermann, S., Hsu, P. D. (2022). Genome-wide bidirectional CRISPR screens identify mucins as host factors modulating SARS-CoV-2 infection. Nature Genetics, 54(8), 1078–1089. https://doi.org/10.1038/s41588-022-01131-x *equal contribution
- Biering, S. B.*, Van Dis, E.*, Wehri, E.*, Yamashiro, L. H.*, Nguyenla, X.*, Dugast-Darzacq, C., Graham, T. G. W., Stroumza, J. R., Golovkine, G. R., Roberts, A. W., Fines, D. M., Spradlin, J. N., Ward, C. C., Bajaj, T., Dovala, D., Schulze-Gamen, U., Bajaj, R., Fox, D. M., Ott, M., Murthy, N., Nomura, D. K., Schaletzky, J., Stanley, S. A. (2021). Screening a Library of FDA-Approved and Bioactive Compounds for Antiviral Activity against SARS-CoV-2. ACS Infectious Diseases, 7(8), 2337–2351. https://doi.org/10.1021/acsinfecdis.1c00017 *equal contribution
- Biering, S. B.*, Akey, D. L.*, Wong, M. P., Brown, W. C., Lo, N. T. N., Puerta-Guardo, H., Tramontini Gomes de Sousa, F., Wang, C., Konwerski, J. R., Espinosa, D. A., Bockhaus, N. J., Glasner, D. R., Li, J., Blanc, S. F., Juan, E. Y., Elledge, S. J., Mina, M. J., Beatty, P. R., Smith, J. L., & Harris, E. (2021). Structural basis for antibody inhibition of flavivirus NS1-triggered endothelial dysfunction. Science, 371(6525), 194–200. https://doi.org/10.1126/science.abc0476 *equal contribution
- Furlong, K.*, Biering, S. B.*, Choi, J., Wilen, C. B., Orchard, R. C., Wobus, C. E., Nelson, C. A., Fremont, D. H., Baldridge, M. T., Randall, G., & Hwang, S. (2020). CD300LF Polymorphisms of Inbred Mouse Strains Confer Resistance to Murine Norovirus Infection in a Cell Type-Dependent Manner. Journal of Virology, 94(17), e00837-20. https://doi.org/10.1128/JVI.00837-20 *equal contribution
- Wang, C.*, Puerta-Guardo, H.*, Biering, S. B.*, Glasner, D. R., Tran, E. B., Patana, M., Gomberg, T. A., Malvar, C., Lo, N. T. N., Espinosa, D. A., & Harris, E. (2019). Endocytosis of flavivirus NS1 is required for NS1-mediated endothelial hyperpermeability and is abolished by a single N-glycosylation site mutation. PLoS Pathogens, 15(7), e1007938. https://doi.org/10.1371/journal.ppat.1007938 *equal contribution
- Puerta-Guardo, H.*, Glasner, D. R.*, Espinosa, D. A., Biering, S. B., Patana, M., Ratnasiri, K., Wang, C., Beatty, P. R., & Harris, E. (2019). Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Reports, 26(6), 1598-1613.e8. https://doi.org/10.1016/j.celrep.2019.01.036 *equal contribution
- Bouziat, R.*, Biering, S. B.*, Kouame, E., Sangani, K. A., Kang, S., Ernest, J. D., Varma, M., Brown, J. J., Urbanek, K., Dermody, T. S., Ng, A., Hinterleitner, R., Hwang, S.#, & Jabri, B#. (2018). Murine Norovirus Infection Induces TH1 Inflammatory Responses to Dietary Antigens. Cell Host & Microbe, 24(5), 677-688.e5. https://doi.org/10.1016/j.chom.2018.10.004 *equal contribution #co-corresponding authors
- Biering, S. B.*, Choi, J.*, Halstrom, R. A., Brown, H. M., Beatty, W. L., Lee, S., McCune, B. T., Dominici, E., Williams, L. E., Orchard, R. C., Wilen, C. B., Yamamoto, M., Coers, J., Taylor, G. A., & Hwang, S. (2017). Viral Replication Complexes Are Targeted by LC3-Guided Interferon-Inducible GTPases. Cell Host & Microbe, 22(1), 74-85.e7. https://doi.org/10.1016/j.chom.2017.06.005 *equal contribution
- Biering, S. B., Huang, A., Vu, A. T., Robinson, L. R., Bradel-Tretheway, B., Choi, E., Lee, B., & Aguilar, H. C. (2012). N-Glycans on the Nipah virus attachment glycoprotein modulate fusion and viral entry as they protect against antibody neutralization. Journal of Virology, 86(22), 11991–12002. https://doi.org/10.1128/JVI.01304-12
Biography
Scott Biering is an Assistant Professor in the Department of Molecular Biology in the School of Biological Sciences at UCSD. Scott received undergraduate degrees from UCLA where he worked in the laboratories of Dr. Benhur Lee and Dr. Hector Aguilar-Carreno investigating entry mechanisms of Nipah virus. He then received his Ph.D. in Microbiology from the University of Chicago in the laboratory of Dr. Seungmin Hwang investigating antiviral mechanisms of interferons and autophagy proteins. He pursued postdoctoral studies in the laboratory of Dr. Eva Harris as an Open Philanthropy Fellow of the Life Sciences Research Foundation at the University of California, Berkeley, investigating mechanisms of flavivirus and coronavirus pathogenesis. He joined the Molecular Biology faculty at UCSD in 2023.

