Nerve Endings in Brain Wiring Found to ‘Talk’ to Each Other
Neurobiologists reveal for the first time that growth cones communicate via a gene found in Parkinson’s disease
July 8, 2020
By Mario Aguilera
The intricate and organized ways in which nerve fibers (axons) are wired in our nervous system are essential to healthy development and function. The critical navigation hubs at the tips of our nerves, the “growth cones,” play a central role in the development of these axonal networks. Scientists have known that individual axonal growth cones are guided by directional cues as they grow, but whether groups of growth cones communicate directly with each other to coordinate their navigation has been unclear.
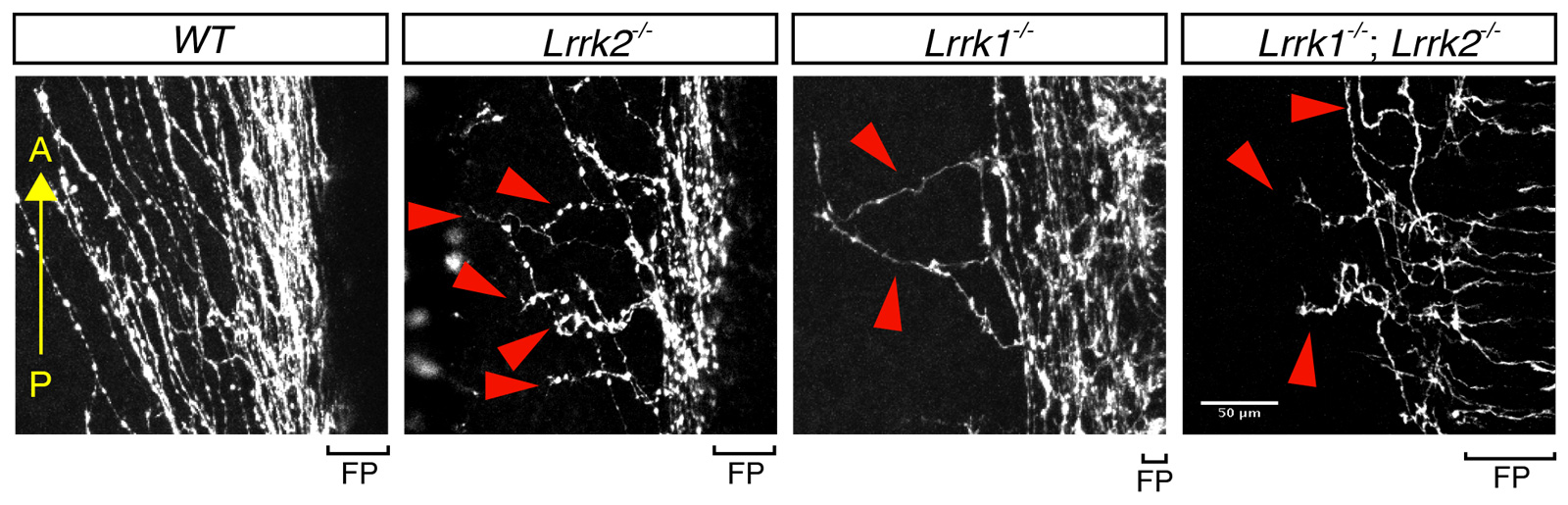
Compared with the trajectory of normal axon growth (wildtype, or “WT”), spinal cord axons missing either or both of the LRRK genes are disorganized and reveal abnormal growth direction.
New findings reported on July 8 in the Proceedings of the National Academy of Sciences solved this long-standing mystery. For the first time, researchers demonstrated that growth cones do indeed communicate with each other and apparently do so to increase the fidelity of wiring.
University of California San Diego postdoctoral scholar Keisuke Onishi, Neurobiology Professor Yimin Zou and their colleagues found that as growth cones talk to one another, they increase their sensitivity to the instruction of guidance molecules.
Further, the researchers found that how they communicate proved fascinating.
“We found a highly conserved signaling pathway, called the planar cell polarity pathway, is essential for regulating this growth cone-growth cone interaction,” said Zou. “The planar cell polarity pathway is known to polarize tissues by cell-cell communication. What is fascinating is that the neuronal growth cones use exactly the same signaling pathway to communicate and navigate as a group.”
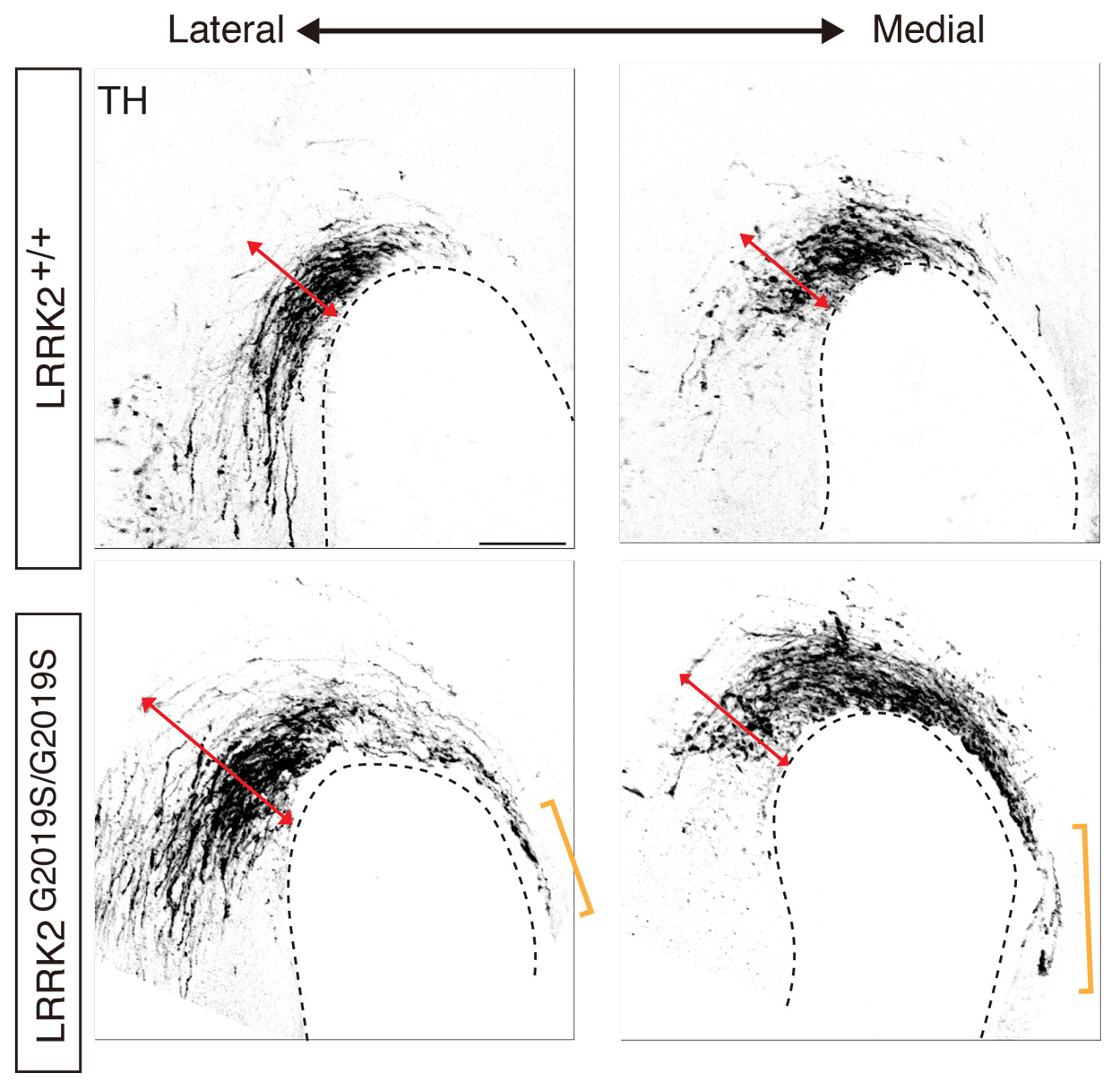
Abnormal development of the midbrain dopaminergic axons in mouse embryos carrying the Parkinson’s disease-causing LRRK2 G2109S mutation.
Growth cone-growth cone communication, the researchers found, is facilitated through the interaction of proteins in the planar cell polarity pathway, such as Fizzled3 and Vangl2. Strikingly, their interaction is regulated by LRRK2, a well-known gene in Parkinson’s disease.
In LRRK2 mutant mice, the Zou lab observed guidance defects of axons conveying body sensations from the spinal cord to the brain as well as the axons of specific neurons that selectively degenerate in Parkinson’s disease. This finding suggests a possible developmental root for this devastating neurodegenerative disorder, as similar developmental defects in human patients carrying LRRK2 mutations may occur. Additional follow-up work, Zou said, is worthwhile to fully explore these clues, as the discoveries may help understand the pathogenesis of Parkinson’s disease and develop potential new therapies.
This work was supported by the National Institutes of Health (grant R37 NS047484).
