UCSD Biologists Discover that Machinery for Cell Division Plays Dual Role in Partitioning Developing Embryo
January 31, 2002
By: Kim McDonald
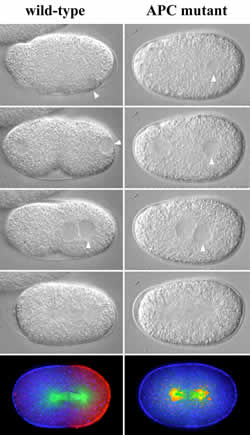
Images of a normal embryo (left) and APC-impaired embryo (right)
Chad Rappleye
Biologists at the University of California, San Diego have discovered that the embryonic development of the first axis of an animal—which defines its inner and outer layers and is initiated by the entry of sperm into an egg—is intimately linked to a protein complex long known to be instrumental in cell division.
Their finding, detailed in a paper featured on the cover of the February issue of the journal Developmental Cell, provides a more complete molecular picture for developmental biologists of how the one-celled embryo divides into an organism’s outer layers—such as its skin and nervous system—and its inner layers, such as its muscle, gut and reproductive organs.
“What we discovered are some of essential parts of the machinery that a cell uses to set up differences in the embryo so that different types of tissue eventually develop,” says Chad A. Rappleye, a graduate student in UCSD’s Division of Biology and the first author of the report. “People have long known that the sperm’s entry into the egg prompts these initial differences. But how the cell sets up these molecular differences has been largely unknown. We found that this protein complex, long associated with a very different function, is essential for positioning the different molecular markers within the cell.”
This protein complex—called Anaphase-Promoting Complex, or APC—appears to play two completely different roles in the cell. APC has long been known among biologists, as its name suggests, for its role in preparing the cell for division by allowing it progress through the metaphase to anaphase transition in the cell cycle. But it now appears to also play a central role in embryonic development.
“Our discovery helps to potentially explain why APC is such a huge complex, because it has multiple roles,” says Raffi V. Aroian, an assistant professor of biology at UCSD who headed the investigation. “What evolution has done is taken a complex process for progression through the cell cycle and used it again to divide the one-celled embryo into its two basic fates, the inside and outside of organisms.”
The UCSD research team—which included Akiko Tagawa, a graduate student in Aorian’s laboratory; Bruce Bowerman, a professor of biology at the University of Oregon; and Rebecca Lyczak, a postdoctoral fellow in Bowerman’s laboratory—uncovered the role of APC in development through a series of painstaking experiments that Rappleye conducted with mutants of the roundworm C. elegans.
Over a two-year period, Rappleye found mutants with impaired APC function and demonstrated that the protein complex helps the sperm push a protein known as PAR-3 away from just one region of the embryo so that a different protein, known as PAR-2, can bind to that one region. In this way, PAR-3 ends up at one end and PAR-2 ends up at the other end of the embryo, establishing fundamental differences from one end to the other so that the one-celled embryo divides into an unequal sized pair of cells. The larger daughter develops into the outer layers of the organism and the smaller into the inner layers. The proteins are known as PAR, because mutations in the genes that produce them lead to errors in the partitioning of germline granules that, in a healthy embryo, all flow into the smaller of the first two dividing cells.
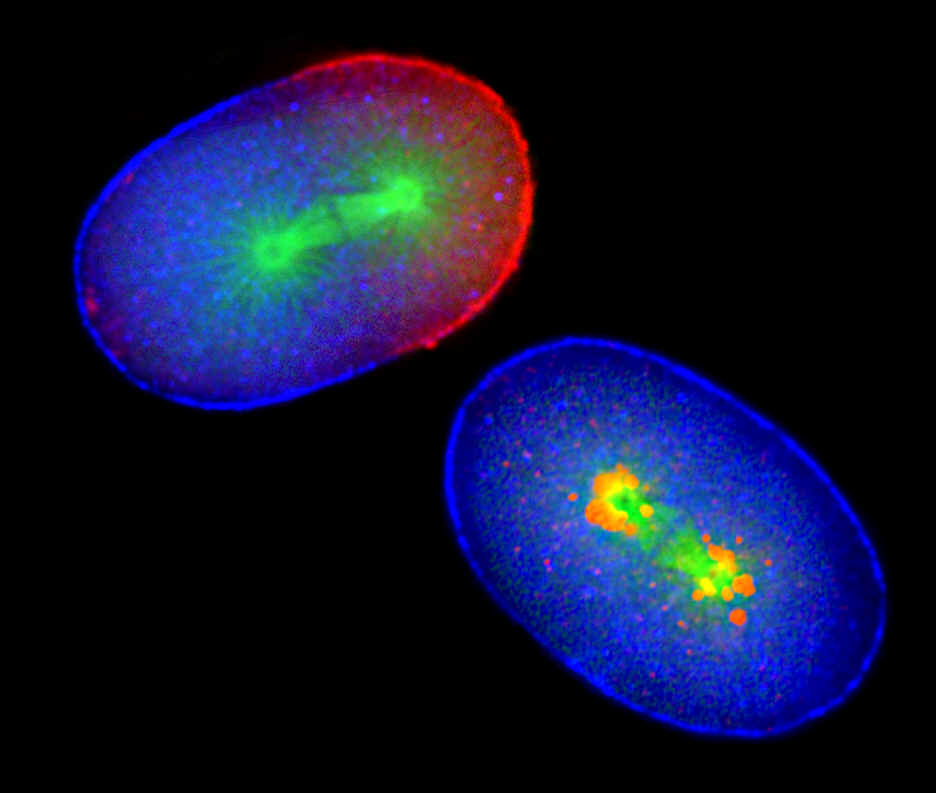
This can be seen in a series of photographs taken by Rappleye of roundworm sperm DNA (at arrow) entering a normal egg (at left) and an egg with impaired APC function (at right). The APC in the normal embryo pushes the PAR-3 proteins in blue) away from the site of the sperm’s entry, resulting in an asymmetric distribution of the PAR-3 and PAR-2 proteins (in red), with the mitotic spindle (in green) displaced toward the PAR-2 end. When APC function is impaired in the mutant embryo (right), the cell splits into two equal sized cells and the mitotic spindle remains at the center.
Besides providing developmental biologists with a better understanding of the biochemistry and genetics of the earliest stages of embryonic development, the UCSD team’s discovery is likely to spur scientists to search for other proteins in the cell with dual roles.
“The idea that the general cellular machinery can play a direct role in specialized processes in development opens up a new way of thinking,” says Aroian. “It changes the mindset of cell and developmental biologists. But from the cell’s perspective, it makes perfect sense. Cells don’t have to develop a whole new type of cellular machinery for development. They can use something they already have for another purpose.”
