Biologists Discover Key To Blocking Inflammation
MARCH 6, 2003
By: Kim McDonald
Biologists at the University of California, San Diego have discovered that eliminating the ability of white blood cells to respond to low oxygen levels effectively blocks the development of inflammation in mice, an advance that could have widespread implications for the prevention of inflammation in humans.
Their discovery, detailed in the March 7 issue of the journal Cell, could lead to the development of a new class of drugs for treating the debilitating and painful joint inflammation in the 43 million Americans who suffer from arthritis. It also may help doctors treat cancer more effectively, because tumor development is associated with a pronounced inflammatory response in the vast majority of cases.
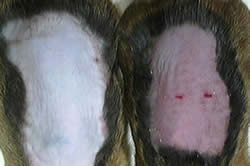 |
| Inflammation response in a normal mouse (reddish skin, at right), compared to mouse lacking HIF-1 (with blocked inflammation response) Credit: Thorsten Cramer, UCSD |
Scientists had previously documented the mechanisms by which white blood cells are directed to sites of injury by the low oxygen levels typically found at those locations. "When you have a cut, the local vascular structure surrounding the wound is disrupted and there's no longer a normal supply of oxygen being delivered," says Randall S. Johnson, an associate professor of biology, who headed the study. "White blood cells (leukocytes) constitute the first line of defense against invading microorganisms like bacteria or viruses and accumulate at sites of injury. Because these cells operate outside of the blood vessels, they need to be able to function in a low-oxygen environment."
Leukocytes that enter these low oxygen, or hypoxic, environments also possess molecular and genetic "switches" that allow them to change their metabolism from an oxygen-dependent (aerobic) to an oxygen-independent (anaerobic) mode of generating energy. But until now, the importance of those switches to regulate inflammation was not well understood. By eliminating the ability of the white blood cells-specifically macrophages and neutrophils-to turn on their hypoxic response and generate energy anaerobically, the UCSD scientists were able to stop the usual development of inflammation in laboratory mice.
"By altering the ability of these cells to operate normally in a low oxygen environment, we've essentially prevented inflammation in mice," notes Johnson.
To demonstrate that this was the case, Johnson and Thorsten Cramer, a gastroenterologist from Berlin in Germany working as a research fellow in Johnson's UCSD laboratory, produced three different strains of mice, each of which had been genetically modified without a key factor in a biochemical pathway known to regulate the ability of the cells to adapt to low oxygen environments.
Testing the responses of the three different strains, the scientists discovered that inactivation of a protein known as hypoxia inducible transcription factor-1, or HIF-1, strongly blocked the inflammatory response in mice. Johnson and Cramer were able to show that HIF-1 is essential for metabolism and energy generation of the white blood cells. In mice without a regulatory protein known as the von Hippel Lindau factor, or VHL, which degrades HIF-1 in normal cells, the UCSD scientists discovered that the inflammatory response was heightened, presumably due to the large excess of HIF-1 that accumulated in the white blood cells.
"Chemists characterized the central importance of anaerobic energy generation for the function of white blood cells almost a century ago," says Cramer. "By using genetic technology we were not only able to confirm these experiments, we also identified a key molecular player involved in white blood cell energy synthesis. To me this is one of the most exciting details about our study."
With the help of Yuji Yamanishi, Gary Firestein and Maripat Corr of the UCSD School of Medicine, the researchers also found that mice without HIF-1 could avoid the joint swelling and inflammation symptoms that typically occur when normal mice are subjected to an arthritis-inducing treatment.
But the loss of HIF-1 does not come without a cost. Experiments conducted in collaboration with Victor Nizet of UCSD's School of Medicine showed that the loss of HIF-1 from white blood cells erodes their ability to kill bacteria. The scientists also found that the ability of the cells to leave the bloodstream and move through the body was significantly impaired without HIF-1. Other scientists who contributed to the study included Bjorn Clausen of the University of Amsterdam in the Netherlands, Irmgard Forster of the Technical University of Munich in Germany, Rafal Pawlinski and Nigel Mackman of the Scripps Research Institute, Volker Haase of the University of Pennsylvania School of Medicine, Rudolf Jaenisch of MIT, and Hans-Peter Gerber and Napoleone Ferrara of Genentech, Inc.
Johnson says one advantage HIF-1 inhibition might have over methods for treating severe arthritic inflammation that involve reducing a patient's white blood cell count, is that blocking HIF-1 doesn't affect the number of circulating white blood cells.
"This may prompt people to take a look at the large number of HIF-1 inhibitors being developed for anti-cancer treatment," he says. "These drugs may turn out to have an even bigger market in treating inflammation and arthritis."
Johnson notes that the discovery may have its greatest value as an insight into the basic biology of cells, which all possess the ability to switch between aerobic and anaerobic metabolism, and cells that circulate through the body.
"Cells that migrate throughout the body need to have the capacity to function in low oxygen environments," he adds. "If you remove that, you've limited the ability of the cell to function. But by blocking this pathway, you've also blocked unwanted inflammation." The study was financed by the National Institutes of Health and the Deutsche Forschungsgemeinschaft (German Research Foundation).
