Slime Mold Reveals Clues to Immune Cells’ Directional Abilities
May 26, 2016
By Kim McDonald
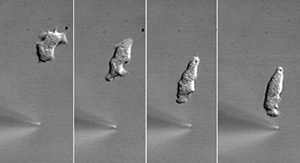
Sequential images, from left to right, of a Dictyostelium cell migrating towards a chemical cue emitted from the needle.
Susan Lee, UC San Diego
How white blood cells in our immune systems home in on and engulf bacterial invaders—like humans following the scent of oven-fresh pizza—has long been a mystery to scientists.
But biologists from UC San Diego and the University of Groningen in the Netherlands have uncovered important clues about this mechanism from an organism commonly encountered in soil, but often unnoticed: the slime mold Dictyostelium discoideum.
The scientists report in this week’s issue of the journal Developmental Cell that the key to the directional ability of this microscopic organism, affectionately known to biologists as “Dicty,” is a protein that when activated by chemicals secreted by bacteria allows the slime mold to home in on and feed on bacteria living in soil.
“Like white blood cells, Dicty has the ability to distinguish and respond differently to a variety of chemical cues, and a sense of direction,” said Richard Firtel, a professor of biology at UC San Diego who co-headed the research collaboration with Arjan Kortholt from the University of Groningen. “When a white blood cell encounters a chemical signal, the chemical binds to proteins, called receptors, on the surface of the white blood cell. These receptors are evenly distributed over the surface of the cell, but the cell has a remarkable ability to identify the type of chemical and the direction from which it originated.”
“The white blood cell then rearranges the internal cellular machinery that allows it to crawl, often over great distances, toward the source of the chemical where it devours the bacteria and wards off infection. While the signals and their receptors and the machinery that allows a cell to crawl were known, the link that determines the type of response and that gives a cell a sense of direction was a mystery.”
Firtel and his colleagues discovered that a protein called “GflB” is most often found “floating” inside the Dicty cell in a folded-up and inactive state. When chemical signals from bacteria bind to a receptor on the surface of Dicty, they discovered, the receptor recruits GflB, which then communicates this information to locally assemble the crawling machinery. Since the chemicals reach the cells as a wave with the highest amount on the side facing the chemical source, the researchers found, GflB is also most active on this side and forms a “leading edge” that orients movement toward the source.
“Similar processes of directed cell movement using chemical cues are hijacked by metastasizing cancer cells to migrate to and invade distant sites in a human body,” said Firtel. “Since GflB and its interacting proteins are found in all species, including humans, our findings provide a keystone to understanding how cells know the direction in which they need to migrate. Thus, understanding how this process works allows us to understand how our immune system functions and also provides the necessary information to develop effective cures for a range of diseases, including cancer.”
Other authors of the paper, in addition to Firtel and Kortholt, were Jesus Lacal of UC San Diego; Youtao Liu, Fabrizia Fusetti and Peter van Haastert of the University of Groningen; and Douwe Veltman of the MRC Laboratory of Molecular Biology in Cambridge, UK.
The research project was supported in part by grants from the U.S. Public Health Service (R01 GM037830 and P01 GM07586).
