UCSD Biologists Identify Genetic Mechanisms Conferring Resistance To 'Bt Toxins'
AUGUST 2, 2001
By: Kim McDonald
Biologists at the University of California, San Diego have discovered the genetic and molecular means by which roundworms, and probably insects, can develop resistance to the most widely used biologically produced insecticide—crystalline toxins from the bacterium Bacillus thuringiensis, or Bt.
Such Bt toxins, which are safe to humans and other vertebrates and far more environmentally friendly than pesticides, have been sprayed on crops by organic farmers for decades. They have also played an important role in Africa in controlling insects that carry disease and are now being used in genetically modified corn, cotton and other crops to control caterpillars and beetles. But as the use of Bt toxins expands worldwide, scientists fear their long-term effectiveness will be threatened by the development of Bt-resistant strains.
The achievement by the UCSD biologists, reported in the August 3 issue of Science, provides important molecular and genetic information that will help scientists develop strategies to delay or circumvent the evolution of Bt-resistant strains of roundworms and insects.
“There are insects in the wild now that contain gene variants that allow them to be resistant to Bt toxins, but fortunately they are small in number,” says Raffi V. Aroian, an assistant professor of biology at UCSD who headed the study. “However, as more crops with Bt genes are planted, it’s only a matter of time before populations of Bt-resistant insects grow numerous enough to become economically troublesome to farmers hoping to control these insects.”
In their study, the researchers examined mutant genes they discovered in the roundworm C. elegans that confer resistance to a particular Bt toxin known as Cry5B. Joel S. Griffitts, a graduate student at UCSD and the lead author of the study, cloned one of these five mutant genes, which the scientists named bre for Bt resistance, then compared differences in the proteins produced by the mutant gene and the corresponding normal gene. That comparison allowed the UCSD researchers, which included postdoctoral fellow Johanna L. Whitacre and technician Daniel E. Stevens, to conclude that the roundworm’s Bt toxin resistance resulted from the loss of a galactosyltransferase, an enzyme that adds carbohydrates to proteins and lipids.
Their discovery prompted the scientists to hypothesize that crystalline Bt toxins—which act by attacking and dissolving the intestines of their hosts—normally recognize the outer surface of intestinal cells by means of carbohydrates or sugars. When the galactosyltransferase gene is missing, these sugars are not made and the toxin fails to recognize its host.
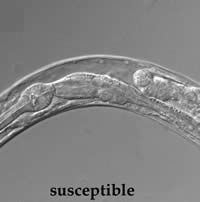 |
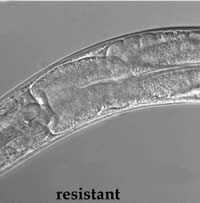 |
| Resistant roundworms fed Bt toxin show no damage to internal structures, unlike the susceptible form. | |
Whether this enzyme is essential for many other Bt toxins remains to be determined. But the UCSD scientists discovered that their mutant roundworms were also resistant to a Bt toxin that is lethal to beetles, suggesting that the development of resistance by the loss of carbohydrate-modifying enzyme is relevant to insects as well. Furthermore the three dimensional structure of diverse insecticidal Bt toxins contains a fold that is predicted to bind precisely the sugar modification made by the galactosyltransferase, raising the possibility that this mechanism of resistance could be widespread.
The discovery that the loss of a general modifier like a galactosyltransferase can allow an organism to develop resistance to Bt toxin is not good news.
“For people using Bt toxins to control insects, this is a particularly threatening scenario,” says Aroian. “The reason is that with one swoop, you can knock out the binding of multiple toxins to multiple receptors. But now that we know this mechanism of resistance, we can devise strategies to cope with this.”
One possible strategy, Aroian says, is for scientists to modify the toxins such that they can bind to the inner lining of the insects’ or roundworms’ guts independent of this carbohydrate modification.
In their study, the UCSD scientists showed visually, using toxins labeled with fluorescent dyes fed to normal and resistant forms of C. elegans, that the Bt toxin is taken up into the gut cells of a normal roundworm but not a resistant roundworm. If the toxin is not recognized, as is the case in resistant animals, it simply passes through the lumen of the gut and is defecated without entering the gut cells.
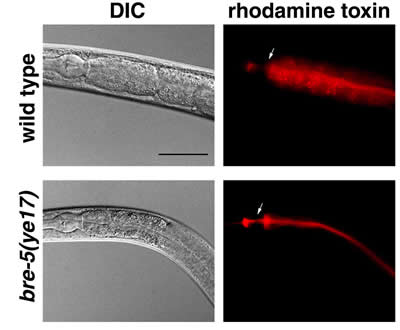 |
| In the wild-type, or normal, roundworms, the Bt toxin (shown in red) readily moves into the gut cells. In the resistant roundworms, the toxin remains in the lumen and is soon eliminated. |
“This provides strong evidence for our model, which essentially is that if you don’t have this carbohydrate enzyme, you don’t make a carbohydrate that the toxin needs to recognize the surface of the gut,” says Aroian. “We also provide, using ‘mosaic analysis,’ definitive molecular evidence that Bt toxins target the gut. Scientists have long known that these toxins targeted the gut. But this, at a molecular level, conclusively proves it.”
The UCSD team’s discovery also sheds light on the puzzling and sometimes contradictory findings of previous attempts to pinpoint a mechanism for the development of Bt resistance in insects.
“ For a couple of decades now,” says Griffitts, the senior author of the paper, “researchers have been grinding up insect guts and finding components of those extracts that stick to Bt toxins. And over the last decade, they’ve found multiple proteins, some of which appear unrelated, that bind to Bt toxins. This study may explain those seemingly contradictory results. These proteins, which may look very different structurally, may have the same binding motif because of carbohydrate modification.”
The discovery of this motif, or mechanism of action, in C. elegans demonstrates the many advantages of this roundworm to researchers. “The kind of analysis that can be done in C. elegans can’t be done as easily in insects,” says Aroian. “We have a complete genetic and physical map of C. elegans, we can breed them in the laboratory easily, they grow fast, having only a three-and-a-half day life cycle, they’re transparent, so we can easily see their internal structures and they eat bacteria, so we can express the Bt toxin right in their food source.”
The discovery of the resistance mechanism in C. elegans will not only help farmers control insects. It will also help scientists employ Bt toxins in the growing problem of roundworm, or nematode, infestations in plants, animals and humans.
“Even if Bt toxins weren’t used to fight insects, nematodes are a huge problem,” says Aroian. “At last estimate, which was 13 years ago, they caused $80-billion worth of crop damage per year. And the damages will become worse, because the main chemical now used to control them in agriculture, methyl bromide, has been banned by the Montreal protocol. They are also a human health problem—a quarter of the world’s population are infected with animal parasitic nematodes.”
The UCSD study was supported by the National Science Foundation, the Burroughs Wellcome Fund and the Beckman Foundation.
