Method To Visualize Gene Activity May Provide Important Insight Into Normal Development And Genome Function
August 5, 2004
By: Sherry Seethaler
A technique developed by University of California, San Diego biologists, which uses bright fluorescent dyes to reveal the activity of genes in individual cells of an organism, promises to be a boon to developmental biologists, and may provide new insight into how cancerous tumors begin and grow.
The advance, described in the August 6 issue of Science, allows researchers, for the first time, to simultaneously visualize the activity of multiple genes in the same cell. The combination of genes that are active in a particular cell during development determines that cell's fate—what type of cell it becomes. The advance also makes it possible to quantify how active a gene is, and even infer the genetic makeup of an organism.
"In addition to facilitating our own research on fruit fly development, there are many potential applications for this technique," says Ethan Bier, a professor of biology at UCSD who led the research team. "For example, it could be used to understand how tumors arise and grow, by revealing what genes are turned on and when. With this information, it should be possible for cancer biologists to predict how aggressive a tumor will be from its early patterns of gene expression."
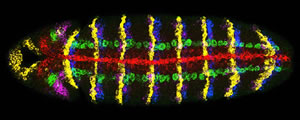
Activity of seven genes in a Drosophila embryo. Credit: Dave Kosman, UCSD
"Cell fate decisions must be understood in order for any of the incredible medical potential of stem cell therapy to be realized," adds Dave Kosman, a research scientist in the Bier and McGinnis laboratories and lead author on the paper
Multiplex labeling, as the technique is called, uses RNA tagged with a fluorescent molecule to signal that a gene is turned on. When a gene is "on" it produces RNA copies–gene transcripts‐of itself. The biologists designed fluorescently-tagged RNA molecules that are complementary to the gene transcripts, and bind to them like Velcro. Therefore a fluorescent beacon reveals the existence and location of the RNA gene copy.
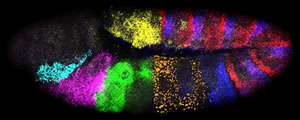
Activity of seven genes in a Drosophila embryo. Credit: Dave Kosman, UCSD
"Multiplex labeling has allowed us to directly map the activation patterns of micro-RNA genes, which were hitherto undetectable," says William McGinnis, a professor of biology at UCSD and co-principal investigator of the study. "Micro-RNAs were known to be important in development, but this is the first evidence indicating that these genes can control the embryonic body plan."
Activity of seven genes in a Drosophila embryo. Credit: Dave Kosman, UCSD
Different colored fluorescent molecules can be used to identify transcripts from different genes in the same cell. It works even if one gene is much more active than another, because the amount of fluorescence of each color is quantified separately.
"When using the microscope to measure the fluorescence, the light is fanned out into a rainbow, and each color is read through a separate channel," explains Bier. "That way if the light is 90 percent blue and ten percent yellow, it might look blue to the naked eye, but the microscope detects each color present."
According to Bier, multiplex labeling fills a gap in developmental biologists' toolkit between gene chips, which can identify several hundred gene transcripts at a time, but not their location, and methods that can reveal the identity and location of up to three gene transcripts simultaneously–though not if they are in the same cell. So far the researchers have used multiplex labeling to visualize the activity of up to seven genes at the same time, but they predict it will be possible to increase this to 50.
Newly developed, ultra-bright fluorescent molecules make the multiplex labeling technique possible. The fluorescent molecules were provided by Molecular Probes, Inc., and the company's scientists also shared their expertise with the UCSD researchers. Developing an effective way to attach the fluorescent molecule to the RNAs complementary to the gene transcripts, and perfecting the overall labeling process were also pivotal in the development of the technique.
"Up until now visualizing gene transcripts has been more art than science," says Kosman. "There was a lot of trial and error involved. We have developed a reliable technique that is powerful enough to generate a molecular fingerprint of the gene activity in a single cell."
Bier contrasted the level of detail revealed with multiplex labeling and previous techniques for visualizing gene activity as being akin to "the difference between looking at the stars through a telescope versus binoculars."The researchers point out that while they have refined the technique in Drosophila embryos, it will likely require modifications to work in other organisms. A detailed guide to the labeling process accompanying the paper, and available through Science's website, should facilitate the necessary adaptations.
Other UCSD contributors to the paper were Claudia M. Mizutani and Derek Lemons and W. Gregory Cox was a contributor from Molecular Probes, Inc. This research was supported by grants from the National Science Foundation and the National Institutes of Health.
