Biologists Use Fruit Flies to Reveal Secrets of How Anthrax Kills
October 18, 2010
By Kim McDonald
Two groups of researchers at UC San Diego have found an explanation for a longstanding mystery of how two very different toxins from anthrax bacteria work together to disrupt essential cell functions during infection with this potential bioterrorism threat.
One group, looking at the effect of anthrax toxins in fruit flies, and the other, examining anthrax in mice and human cells, demonstrate that the two anthrax toxins act in a cooperative fashion to prevent the final step by which cells transport molecules to their surfaces in order to communicate and adhere to one another.
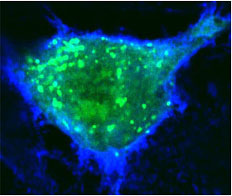
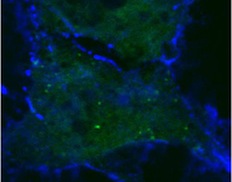
Normal human blood vessel cells (top) are made leaky when both anthrax toxins are present (bottom).
Credit: Annabel Guichard and Nina van Sorge
By interfering with these specialized sites of cell to cell interaction, the UCSD researchers conclude in a paper in the October 14 issue of the journal Nature, that anthrax toxins prevent cells from delivering critical components to sites of cell to cell contact, which is likely to contribute to the failure of blood vessels that kills their victims during the final stages of infection.
Information about how the two anthrax toxins work in concert with one another at the cellular level is becoming increasingly important as researchers devise ways to better protect large populations from the threat of anthrax attacks by bioterrorists.
"We know that one key reason that anthrax kills is by making blood vessels and cell barriers leaky," said Ethan Bier, a professor of biology at UCSD and the senior author of the paper. "But the mystery has been how these dangerous changes could be related to two very different types of toxins the bacteria inject into their hosts?"
Bier and his team of scientists in UCSD's Division of Biological Sciences, specifically Annabel Guichard and Beatriz Cruz-Moreno, gained the first hint in solving that mystery when they found that the two anthrax toxins worked cooperatively in flies to produce abnormal wings with notches along their edges.
"Their experiments revealed that both toxins inhibit transport of molecules required for signaling and adhesion to points of cell to cell contact," said Bier. "By impinging on two pivotal regulators of the cellular mail service, these two toxins effectively eliminated the delivery of critical components to zones essential for cells communicating with each other and forming coherent structures such as the wing margins of flies."
Following up on that discovery, the second group of researchers, headed by Victor Nizet, a professor of pediatrics in UCSD's School of Pharmacy, found that treating human cells with anthrax toxins led to similar defects in transporting molecules to sites of cell to cell contact. Nizet's team of scientists, specifically Shauna McGillivray and Nina Van Sorge, also found in their studies that injection of live anthrax bacteria beneath the skin or into the lungs of mice caused nearby blood vessels to leak, while mutant strains of bacteria lacking the toxins had no such effect.


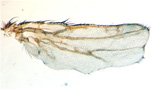
The two anthrax toxins work cooperatively to produce abnormal fly wings with notches (bottom). Normal wing at top: wing with one toxin in middle.
Credit: Annabel Guichard and Beatriz Cruz-Moreno.
"Our studies with human cells and mice suggest that the ability of anthrax toxins to inhibit delivery of adhesive molecules to sites responsible for cell to cell contact leads to leaky blood vessels and may contribute to the final stages of anthrax infection in which blood vessels fail and the patient develops shock, often followed by rapid death," said Nizet.
Bier added that the collaboration between biologists working on invertebrates and biomedical researchers working on mammalian systems "highlights the growing opportunities to make potentially important medical discoveries in simpler and rapid systems such as the fruit fly, and later validate them in systems more directly relevant to the clinical disease process such cultured human cells or the laboratory mouse."
"In future studies, it will be interesting to investigate whether inhibition of the cellular mail circuit by anthrax toxins may also prove to be important in other aspects of anthrax pathogenesis, such as avoiding uptake and killing by immune cells," he added. "One can anticipate that other bacterial and viral pathogens may interfere with the same critical processes during the development of infection."
Funding for the study was provided by grants from the National Institutes of Health.
Related Links