UC San Diego Biologists Discover Second Motor Protein That Rewinds DNA
November 16, 2010
By Kim McDonald
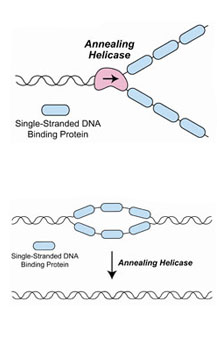
The two annealing helicases are molecular motor proteins that move along the DNA strand to rewind sections of the double-stranded DNA molecule that become unwound in "bubbles," like that above, which interfere with normal cellular functions, including the synthesis and repair of DNA.
Credit: Jim Kadonaga, UCSD
Biologists at the University of California, San Diego have found another member in a new class of cellular motor proteins that rewind and seal together sections of the double-stranded DNA molecule that occasionally but perilously become unwound.
Their discovery, which provides a promising new avenue for the development of drugs that kill cancer cells, is detailed in a paper published in this week's early online edition of the Proceedings of the National Academy of Sciences.
The same biologists two years ago discovered the first of these motor proteins, which they termed "annealing helicases" because they anneal single strands of DNA together like a molecular zipper.
"These two annealing helicases appear to be important for the maintenance of the double-helical structure of our genome, which is about two meters of double-stranded DNA in each human cell," said Jim Kadonaga, a professor of biology at UC San Diego who discovered the two proteins with Timur Yusufzai, a postdoctoral fellow working in his laboratory. "The discovery of this second protein suggests that there may be a family of annealing helicases and that these motor proteins have specialized functions in the cell."
The two annealing helicases are molecular motor proteins that move along the DNA strand to rewind sections of the double-stranded DNA molecule that become unwound in "bubbles" that interfere with normal cellular functions, including the synthesis and repair of DNA.
"DNA is much more stable in its double-stranded form than in its single-stranded form," said Kadonaga. "When your DNA gets stuck in the unwound single-stranded form, your cells are in big trouble, and in humans, that ultimately leads to death. These DNA rewinding enzymes fix this problem and remove the unstable and biologically dangerous single-stranded DNA regions."
The existence of annealing helicases was first discovered two years ago by Kadonaga and Yusufzai in their studies of a protein called HARP for HepA-related protein. They were interested in HARP because mutations in the protein cause a rare genetic disorder called Schimke immuno-osseous dysplasia. The elucidation of the function of the HARP protein has since provided biomedical scientists with a greater understanding of the underlying mechanisms that lead to this devastating genetic disorder, which causes strokes, congestive heart failure, kidney failure and death in young children.
The second motor protein, called AH2 for "annealing helicase 2," was discovered by the scientists looking for more members in the same family of enzymes. The scientists said that the AH2 protein has structural and functional differences from HARP that suggest that different annealing helicases have distinctly different roles in the cell.
Despite their differences in the cell, both of these annealing helicases may be attractive targets for the development of new kinds of anti-cancer drugs.
"Many anticancer drugs target DNA or enzymes that alter DNA structure," said Kadonaga. "These drugs interfere with the normal synthesis of DNA in rapidly growing cancer cells."
"Because AH2 and HARP are the first two members of a new type of DNA-modifying enzyme, they are attractive targets for a new class of anticancer drugs," he added. "Moreover, inhibitors of AH2 and/or HARP could potentially act in synergy with currently used anti-cancer drugs, such as etoposide, doxorubicin and cisplatin, to disrupt DNA synthesis and repair."
"As a result, our discovery of both AH2 and HARP provides not only new scientific insight into the molecular mechanisms involved in the maintenance of genome integrity, but also a potential new avenue for the discovery of new drugs that kill cancer cells."
The researchers' work was supported by a grant from the National Institutes of Health.
They have filed a patent application on their discoveries and their technology (SD2010-140) and related technologies are available for licensing and commercial development through the UCSD Technology Transfer Office.
Related Links- James Kadonaga
- Discovery of first motor protein
- Annealing helicase 2 (AH2), a DNA-rewinding motor with an HNH motif in Proceedings of the National Academy of Sciences
