Researchers Unravel Key Mechanism Underlying Hydra’s Regenerative Ability
Findings describe how animal’s cells self-assemble to become a separate organism
December 19, 2017
By Mario Aguilera
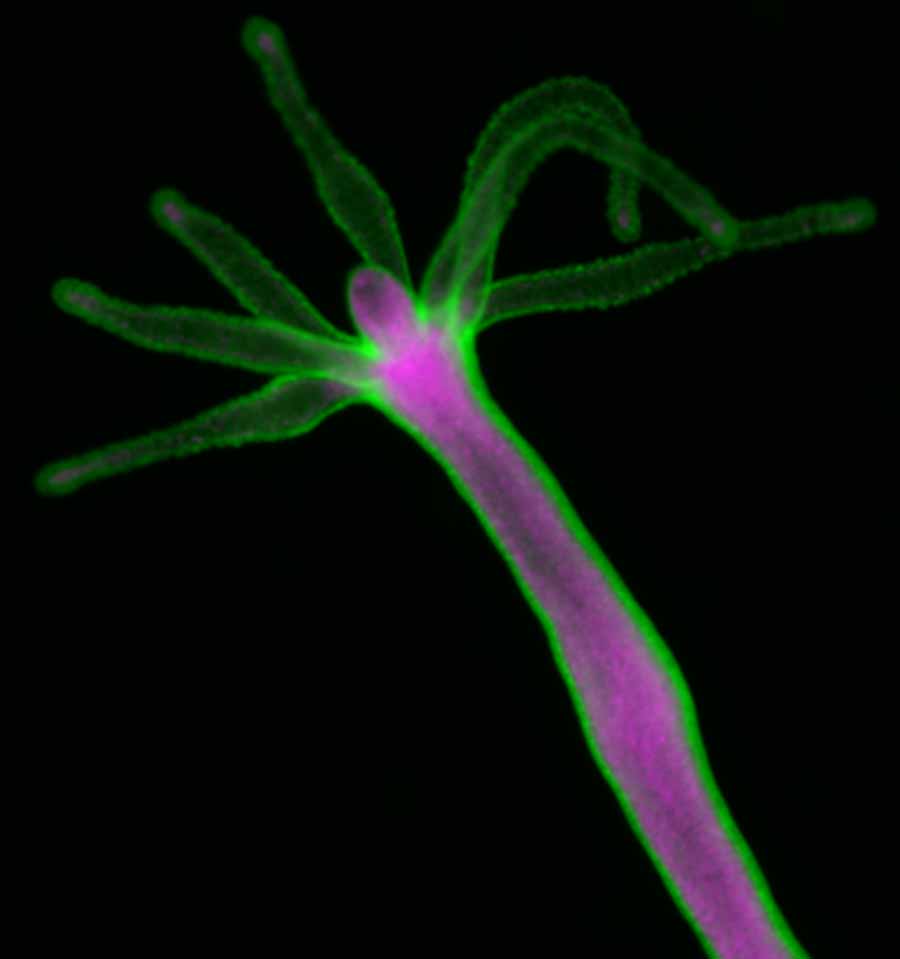
When a watermelon hydra is divided into individual cells, the animal will regenerate with green outer-layer cells and magenta inner-layer cells separating from each other to take up their respective inner and outer positions.
Hydra, a gangly, translucent animal found in freshwater ponds and lakes, has been the subject of countless studies due to its astonishing ability to regenerate. A relative of jellyfish, the multi-tentacled Hydra boasts the uncanny capacity to regenerate into a complete new organism after having been dissociated into individual cells.
Research conducted at the University of California San Diego led by biophysicist Eva-Maria Collins has solved a key mechanism underlying this process by breaking down how Hydra’s cells are able to self-organize into functioning tissue during regeneration.
This process, called “cell sorting,” is carried out in most animals during development but Hydra’s ability to regenerate make it a powerful model to methodically study how the process unfolds. Using a variety of approaches—from fluorescent protein markers to lab experiments to numerical models—the researchers found that Hydra cell sorting can be explained with the same physics as the separation of salad dressing into oil and vinegar. A process called “tissue interfacial tension” drives cells through the sorting process and into assembly in the new organism.
The findings are described in the Dec. 19, 2017 issue of Biophysical Journal.
“How cell sorting works has been debated for a long time so it’s great to finally have been able to combine all these different experimental and theoretical tools to give an answer on the mechanism,” said Collins, who led the research while she was an associate professor in UC San Diego’s Department of Physics and Section of Cell and Developmental Biology. She now has an adjunct appointment at UC San Diego and an associate professorship at Swarthmore College in Pennsylvania.
Future studies will probe the molecular machinery underlying the tissue surface tension that drives cell sorting during regeneration.
The research was carried out by an interdisciplinary team involving UC San Diego Department of Physics postdoctoral researcher Olivier Cochet-Escartin, Biological Sciences master’s student Tiffany Locke, Bioengineering undergraduate student Winnie Shi, as well as Professor Rob Steele from the Department of Biological Chemistry and the Developmental Biology Center at UC Irvine.
The research was supported by the National Science Foundation (grant NSF CMMI-1463572) and the Research Corporation for Science Advancement.
